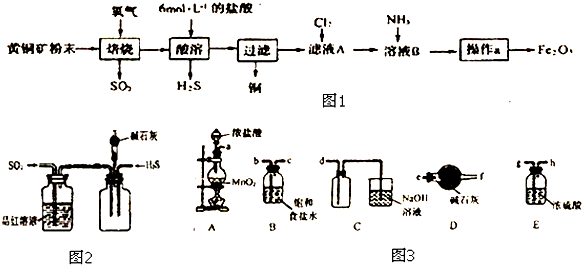
��Ŀ����
9��������������Ni2O3����һ����Ҫ�ĵ���Ԫ�����Ϻ����ز��ϣ���ҵ�����ú������ϣ����������ơ�þ�Ͻ�Ϊ������ȡ��������NiC2O4•2H2O�����ٸ������ղ�������ȡ��������������֪����ĸơ�þ�����ξ�������ˮ���繤������ͼ��ʾ��
��ش��������⣺
��1��������Ϊ�����ܽ⣬���ˣ�
��2�����������ж�ν��й��ˣ�ʵ���ҽ��й��˲�������Ҫ�õ�������������ʵ������в�������������ȫ��ͬ����bc������ĸ����
�ٲⶨ��������Һ��pH �ڼ���ʳ����Һ�Ʊ�NaCl����
������0.1mol/L��������Һ ���õ���-KI��ֽ������Һ������������
������10%����������Һ
a���٢�b���ڢ�c���٢�d���ۢ�
��3���ټ���H2O2��������Ҫ��Ӧ�����ӷ���ʽΪ2Fe2++H2O2+2H+�T2Fe3++2H2O��
�ڼ���̼������Һ��pH��4.0��5.0����Ŀ��Ϊ�ٽ�������ˮ�������ȫ��
�ۼ���NH4F�������������Ca2+��Mg2+��
��4����������NiC2O4•2H2O�����ȿ����и�����ˮ���ڸ��������գ����Ƶ�Ni2O3��ͬʱ��û�����壮���������ȷֽ�Ļ�ѧ����ʽΪ2NiC2O4$\frac{\underline{\;����\;}}{\;}$Ni2O3+3CO��+CO2����
��5����ҵ�ϻ����õ�ⷨ��ȡNi2O3����NaOH��Һ��NiCl2��Һ��pH��7.5����������Na2SO4�����ö��Ե缫��⣮�������в�����Cl2��80%������������������ClO-���ٰѶ���������Ϊ��������ClO-����Ni��OH��2����Ni2O3�����ӷ���ʽΪClO-+2Ni��OH��2�TCl-+Ni2O3+2H2O��a mol������ȫ��ת��Ϊ������ʱ�����·��ͨ�����ӵ����ʵ���Ϊ1.25a mol��
��6����Al��NiO��OH��Ϊ�缫��NaOH��ҺΪ���Һ���һ�����͵�أ��ŵ�ʱ��NiO��OH��ת��ΪNi��OH��2���õ�ط�Ӧ�Ļ�ѧ����ʽ��Al+3NiO��OH��+NaOH+H2O=3Ni��OH��2+NaAlO2��
���� ��1������������Ҫ�����������ơ�þ�Ͻ�Ϊ���������������ܺ���ˣ��ܽ��������ȥ�����
��2���ٲⶨNa2CO3��Һ��pH��������պȡ��Һ����pH��ֽ�ϲⶨ��
�ڼ���ʳ����Һ�Ʊ�NaCl���壬�������������ֲ����ȹ���ɽ���
������O.1mol/L��H2SO4��Һ�������������ã�
����KI������ֽ������Һ�����������ӣ�������պȡ��Һ����KI������ֽ�ϲⶨ��
������10%��KNO3��Һ����������������ܽ⣻
��3����������������������Ϊ�����ӣ����ݵ���غ��ԭ���غ㣬����Na2CO3��Һ��pH��4.0〜4.5���ٽ�������ˮ���γ��������������������ơ�����þ������ˮ��
��4����������NiC2O4•2H2O�����ȿ����и�����ˮ������NiC2O4��NiC2O4�ٷ���������ԭ��Ӧ��Ni��+2�����ߵ�+3�ۣ���C��+3�۽��͵�+2�ۣ�����Ҫ�����ɻ�����壬����һ����ΪCO2������Ni2O3��CO��CO2�������û��ϼ����������ƽ��
��5��Cl��+1�۽��͵�-1�ۣ�Ni��+2�����ߵ�+3�ۣ����û��ϼ�������ȿ���ƽClO-��Ni��OH��2��Cl-��Ni2O3��ϵ����������Hԭ���غ���ƽˮ��ϵ������������Oԭ�Ӽ����ƽ�Ƿ���ȷ�����ݻ�ѧ����ʽ��Ԫ���غ����õ���
��6����Al��NiO��OH��Ϊ�缫��NaOH��ҺΪ���Һ���һ�����͵�أ��ŵ�ʱNiO��OH��ת��ΪNi��OH��2��������ԭ��Ӧ����Al����������Ӧ�����������µõ�NaAlO2����ƽ��д����ʽ��
��� �⣺��1��������ͼ�������ܡ����ɺ����������ơ�þ���ӵ���Һ�������ܽ⣬�ܽ��������ȥ��������ˣ�
�ʴ�Ϊ�������ܽ⣬���ˣ�
��2������KI������ֽ���� ��Һ�����������ӣ�������պȡ��Һ����KI������ֽ�ϲⶨ��
�ڼ���ʳ����Һ�Ʊ�NaCl���壬�������������ֲ����ȹ���ɽ���
������O.1mol/L��H2SO4��Һ�������������ã�
�ܲⶨNa2CO3��Һ��pH��������պȡ��Һ����pH��ֽ�ϲⶨ��
������10%��KNO3��Һ����������������ܽ⣻
���Ԣڢݺ͢٢ܷ��ϣ�
�ʴ�Ϊ��bc��
��3����˫��ˮ��Ŀ������������Fe3+����Ӧ�����ӷ���ʽΪ��2Fe2++H2O2+2H+�T2Fe3++2H2O������̼������Һ�������ǵ���pH���ٽ�������ˮ�������ȫ���ټ���NH4F��Ŀ���dz�ȥ�����ӡ�þ���ӣ������Ӳ���ʱ���ɲ�����������
�ʴ�Ϊ��2Fe2++H2O2+2H+�T2Fe3++2H2O���ٽ�������ˮ�������ȫ��Ca2+��Mg2+��
��4����������NiC2O4•2H2O�����ȿ����и�����ˮ������NiC2O4��NiC2O4�ٷ���������ԭ��Ӧ��Ni��+2�����ߵ�+3�ۣ���C��+3�۽��͵�+2�ۣ�����Ҫ�����ɻ�����壬����һ����ΪCO2������Ni2O3��CO��CO2�������û��ϼ�������ȣ�Ni������2����3-2����C������1����4-3����C�����ͣ�3����3-2������ƽ����ʽΪ��2NiC2O4$\frac{\underline{\;����\;}}{\;}$Ni2O3+3CO��+CO2����
�ʴ�Ϊ��2NiC2O4$\frac{\underline{\;����\;}}{\;}$Ni2O3+3CO��+CO2����
��5��Cl��+1�۽��͵�-1�ۣ�Ni��+2�����ߵ�+3�ۣ����û��ϼ�������ȿ���ƽClO-��Ni��OH��2��Cl-��Ni2O3��ϵ����������Hԭ���غ���ƽˮ��ϵ������������Oԭ�Ӽ����ƽ�Ƿ���ȷ���õ����ӷ���ʽΪ��ClO-+2Ni��OH��2�TCl-+Ni2O3+2H2O��
ClO-+2Ni��OH��2�TCl-+Ni2O3+2H2O
1 2
n��ClO-�� a mol
��n��ClO-��=0.5 a mol
Cl2+2OH-�TClO-+Cl-+H2O
0.8n��Cl2�� 0.5 a mol
��n��Cl2��=0.625 a mol
���е��ʱ��
2Cl--2e-�TCl2��
2 1
n��e-�� 0.625 a mol
��n��e-��=1.25a mol��
�ʴ𰸣�ClO-+2Ni��OH��2�TCl-+Ni2O3+2H2O��1.25a mol��
��6����Al��NiO��OH��Ϊ�缫��NaOH��ҺΪ���Һ���һ�����͵�أ��ŵ�ʱNiO��OH��ת��ΪNi��OH��2��������ԭ��Ӧ����Al����������Ӧ�����������µõ�NaAlO2���õ�ط�Ӧ�Ļ�ѧ����ʽ�ǣ�Al+3NiO��OH��+NaOH+H2O=3Ni��OH��2+NaAlO2��
�ʴ�Ϊ��Al+3NiO��OH��+NaOH+H2O=3Ni��OH��2+NaAlO2��
���� ���⿼�������̷����ƶϣ�ʵ���������ķ����жϣ���ѧ����ʽ����͵��ԭ������Ӧ�ã���Ŀ�ۺ��Խϴ��ѶȽϴ�


| A�� | 40��֮ǰ��40��֮����Һ������ʱ�����¶ȵı仯�����෴ | |
| B�� | ͼ��b��c�����Ӧ��NaHSO3�ķ�Ӧ������� | |
| C�� | ͼ��a���Ӧ��NaHSO3�ķ�Ӧ����Ϊ5.0��10-5 mol•��L•s��-1 | |
| D�� | �¶ȸ���40��ʱ�����۲���������ʵ���ָʾ�� |
| A�� | Fe2O3 | B�� | V2O5 | C�� | MnO2 | D�� | Cr2O3 |
| A�� | HCl��H2SO4�зų�H2��������ͬ | |
| B�� | CH3COOH�зų�H2��������� | |
| C�� | H2SO4�зų�H2��������죬CH3COOH�зų�H2���������� | |
| D�� | H2SO4�зų�H2������࣬�������� |
| A�� | CH2=CH-CI | B�� | CH2=CH-CH=CH2 | C�� | CH3-CH=CH2 | D�� |  |
 p C(g) ��H�����ܱ������н��У���ͼ��ʾ�ڲ�ͬ��Ӧʱ��t ʱ�¶�T��ѹǿP�뷴Ӧ��B�ڻ�������еİٷֺ���B%�Ĺ�ϵ���ߣ������߷����������ж���ȷ����( )
p C(g) ��H�����ܱ������н��У���ͼ��ʾ�ڲ�ͬ��Ӧʱ��t ʱ�¶�T��ѹǿP�뷴Ӧ��B�ڻ�������еİٷֺ���B%�Ĺ�ϵ���ߣ������߷����������ж���ȷ����( )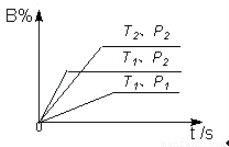
 1��T2��P1��P2��m+n��p����H��0
1��T2��P1��P2��m+n��p����H��0