��Ŀ����
16�����б�ʾ��Ӧ��ѧ��Ӧ�����ӷ���ʽ��������ȷ���ǣ�������| A�� | ��NaAlO2��Һ��ͨ��������CO2��2AlO2-+CO2+3H2O�T2Al��OH��3��+CO32- | |
| B�� | ��NH4��2Fe��SO4��2��Һ�м�����������������Һ��NH4++SO42-+Ba2++OH-�TBaSO4��+H2O | |
| C�� | ��ҵ���ð�ˮ���ն�������2NH3��H2O+SO2�T2NH4++SO32- | |
| D�� | ��������������ϡ����3Fe2++4H++NO3-�T3Fe3++NO��+2H2 |
���� A��������̼������Ӧ����̼�����ƣ�
B�������������������������������ӷ�Ӧ���백�����ӷ�Ӧ��
C����ˮ��������������Ӧ����������泥�
D������������Ϊ������Ӧ������ѧʽ��
��� �⣺A����NaAlO2��Һ��ͨ��������CO2�����ӷ���ʽ��AlO2-+CO2+2H2O=Al��OH��3��+HCO3-����A����
B����NH4��2Fe��SO4��2��Һ�м�����������������Һ�����ӷ���ʽ��NH4++Fe3++2SO42-+2Ba2++4OH-�T2BaSO4��+H2O+Fe��OH��3��+NH3•H2O����B����
C����ˮ��������������Ӧ����������泥����ӷ���ʽ��2NH3��H2O+SO2�T2NH4++SO32-����C��ȷ��
D����������������������ϡ�����е����ӷ�ӦΪ3Fe3O4+NO3-+28H+�T9Fe3++14H2O+NO�������۴���D����
��ѡ��C��
���� ���⿼�������ӷ���ʽ����д�����ؿ��鷴Ӧ��������ͬ������Ӧ��ͬ�����ӷ���ʽ��д����ȷ��Ӧʵ���ǽ���ؼ�����Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
4����������Ϊȼ�ϵ�����ȼ�ϵ�ؽṹ��ͼ��ʾ�����ڸõ�ص�������ȷ���ǣ�������
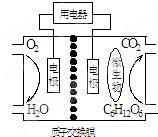

| A�� | �õ���ܹ��ڸ����¹��� | |
| B�� | ��صĸ�����ӦΪ��C6H12O6+6H2O-24e-=6CO2��+24H+ | |
| C�� | �ŵ�����У�H+������������Ǩ�� | |
| D�� | �ڵ�ط�Ӧ�У�ÿ����1mol�����������������ɱ�״��������$\frac{2.42}{6}$L |
5��N2O3���������������һ�������¿���4NO2��g��+O2��g��?2N2O3��g����H��0�ϳɣ�T1��ʱ�������Ϊ2L�ĺ����ܱ�������ͨ��NO2��O2������ʵ���������±���
����˵������ȷ���ǣ�������
| ʱ��/s | 0 | 5 | 10 | 15 |
| c��NO2��mol/L | 4.00 | 2.52 | 2.00 | c3 |
| c��O2��mol/L | 1.00 | c1 | c2 | 0.50 |
| A�� | 5s��O2�ķ�Ӧ����Ϊ0.074mol/L•s�� | |
| B�� | T1��ʱƽ�ⳣ��Ϊ0.125��ƽ��ʱNO2��O2��ת���ʾ�Ϊ50% | |
| C�� | T1��ʱƽ�ⳣ��ΪK1��T2��ʱƽ�ⳣ��ΪK2����T1��T2����K1��K2 | |
| D�� | �����������䣬�������������ѹ����ԭ����һ�룬�����´ﵽƽ��ʱc��N2O3����2mol/L |
6�����й���AlCl3��˵������ȷ���ǣ�������
| A�� | AlCl3��Һ�л����ܴ������ڣ�H+��NH4+��SO42-��NO3- | |
| B�� | AlCl3��Һ������İ�ˮ��Ӧ�����ӷ���ʽΪ��Al3++4NH3•H2O�T4NH4++AlO2-+2H2O | |
| C�� | ��NAΪ����٤��������ֵ����1L0.1mol•L-1��AlCl3��Һ��Al3+����ĿΪ0.1NA | |
| D�� | ��ҵ�Ͽ��õ�����ڵ�AlCl3����ȡ����Al |


 ��
�� ��
�� ��
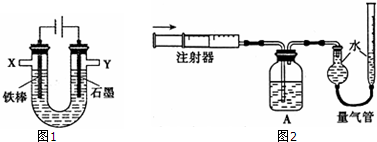
��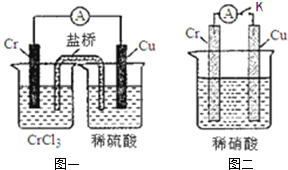 ����ͼװ���У��۲쵽ͼһװ��ͭ�缫�ϲ�����������ɫ���ݣ�����ͼ��װ���е�����K�Ͽ�ʱ�����缫������K�պ�ʱ�����缫�ϲ���������ɫ���壬����ɺ���ɫ���壮
����ͼװ���У��۲쵽ͼһװ��ͭ�缫�ϲ�����������ɫ���ݣ�����ͼ��װ���е�����K�Ͽ�ʱ�����缫������K�պ�ʱ�����缫�ϲ���������ɫ���壬����ɺ���ɫ���壮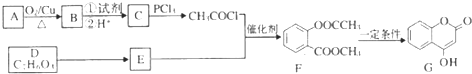

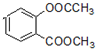 +3NaOH��CH3COONa+CH3OH+
+3NaOH��CH3COONa+CH3OH+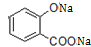 +H2O��
+H2O��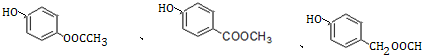 ��д������һ�ֵĽṹ��ʽ����
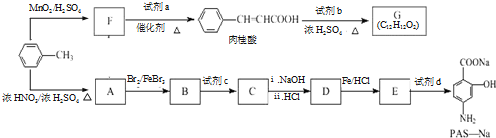
��д������һ�ֵĽṹ��ʽ����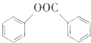 ����һ����Ҫ���л��ϳ��м��壬��д���Ա����ױ�Ϊԭ����ȡ�û�����ĺϳ�·������ͼ����ԭ�����ã�
����һ����Ҫ���л��ϳ��м��壬��д���Ա����ױ�Ϊԭ����ȡ�û�����ĺϳ�·������ͼ����ԭ�����ã�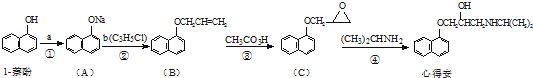
 ����F����һ��������Ļ�ѧ����ʽΪ��
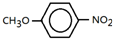
����F����һ��������Ļ�ѧ����ʽΪ�� +HNO3��Ũ��$��_{��}^{Ũ����}$
+HNO3��Ũ��$��_{��}^{Ũ����}$ +H2O�����㻯����F�������ǶԱ������ᣮ
+H2O�����㻯����F�������ǶԱ������ᣮ