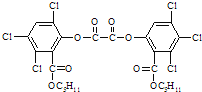��Ŀ����
����Ŀ�����ݹ�����������ί�����ġ�����״�����������Ʒ���(���е��߰�)��ָ������״�����������ߺ������У��������������������֬�ܼ�������Ч�����������Ѷ�������Ч���������Ѷ�����ϴ��̩����ѧ��Ϊ˫�ȱ�˫�����飬ϵ�����ӱ�����Լ��������൱ǿ�Ĺ����־���ɱ�����ã���ṹ��ͼ��ʾ�����й����ȼ�����˵������ȷ����
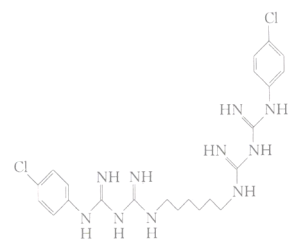
A.�����ʽΪC22H30Cl2N10
B.�䱽���ϵ�һ�����������
C.һ�������£����Ѷ�������6mol�����ӳ�
D.һ�������£����Ѷ��ɷ����ӳɷ�Ӧ��������Ӧ
���𰸡�A
��������
A��������ṹ��ʽ��֪���Ѷ��ķ���ʽΪC22H30Cl2N10����A��ȷ��
B�������ȼ����Ľṹ��ʽ�������������ڶԳ�λ�ã������䱽���ϵ�һ�����ֻ�����֣���B����
C�����Ѷ��Ľṹ������������������6 mol�����ӳɣ�������ṹ�л���4��C=N������������10 mol�����ӳɣ���C����
D�����Ѷ��в����ǻ���Ҳ�����Ȼ������ܷ���������Ӧ��D�����
�ʴ�ΪA��

��ϰ��ϵ�д�
 ���������������Բ��������ϵ�д�
���������������Բ��������ϵ�д�
�����Ŀ