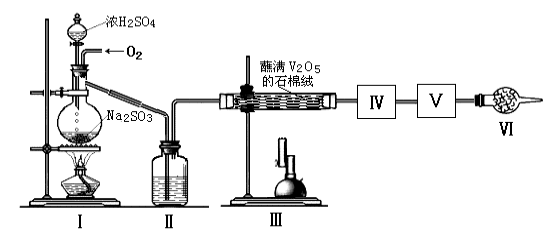
��Ŀ����
����Ŀ����֪A��B��C��D��E��F��G�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����������������A��B��C��D��EΪ��ͬ�����Ԫ�ء�A��C��������������������Ӳ�����2����B�ĵ縺�Դ���C������ɫ�ܲ����۲�E����ɫ��ӦΪ��ɫ��F�Ļ�̬ԭ������4��δ�ɶԵ��ӣ�G��+1�����������ó���K��L��M�������Ӳ㡣�ش��������⣺
��1��A��B��C��D��E��F��G����Ԫ���е�һ��������С��������Ԫ�ط�������DԪ�ص�ԭ�Ӻ������ֲ�ͬ�˶�״̬�ĵ��ӣ����ֲ�ͬ�ܼ��ĵ��ӡ���̬��F3+��������Ų�ʽ�ǡ�
��2��B����̬�⻯����ˮ�е��ܽ��Զ����A��C����̬�⻯�ԭ���ǡ�
��3��������ECAB�е���������AC2��Ϊ�ȵ����壬�������ӵĵ���ʽ�ǡ�
��4��FD3��ECAB��Һ��ϣ��õ�������������Ѫ��ɫ��Һ��������λ��Ϊ5�������Ļ�ѧʽ�ǡ�
��5��������EF[F��AB��6]��һ����ɫ���壬��ͼ��ʾ�侧����1/8��E+δ������������ɫ�����һ��������E+�ĸ���Ϊ��
��6��G�Ķ��������������Ҷ�����H2N��CH2һCH2��NH2���γ������ӣ�
![]()

���������к��еĻ�ѧ�������С�������ĸ��
a�����
B�����Լ�
C�����Ӽ�
D���Ǽ��Լ�
������CAB-�е�Aԭ�����Ҷ�����H2N��CH2һCH2��NH2����Cԭ�ӵ��ӻ���ʽΪ��
���𰸡���1��K1751s22s22p63s23p63d5
��2��NH3��H2O���Ӽ���������CH4��H2S������H2O���Ӽ䲻�������
��3��[![]() ]-
]-
��4��K2Fe��SCN��5
��5��4��6��abdspsp3
��������
���������A��B��C��D��E��F��G�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵������������A��B��C��D��EΪ��ͬ�����Ԫ�أ�A��C��������������������Ӳ�����2����AΪC��CΪSԪ�أ�����ɫ�ܲ����۲�E����ɫ��ӦΪ��ɫ����EΪKԪ�أ�Dԭ����������C��С��EΪ����Ԫ�أ���D��ClԪ�أ�B�ĵ縺�Դ���C����ԭ������С��C��������Ԫ�ش��ڲ�ͬ���壬��BΪNԪ�أ�F�Ļ�̬ԭ������4��δ�ɶԵ��ӣ���λ�ڵ�������Ԫ�أ���FΪFeԪ�أ�G��+1�����������ó���K��L��M�������Ӳ㣬��GΪCuԪ�أ�
��1��Ԫ�صĽ�����Խǿ�����һ������ԽС���⼸��Ԫ���н�������ǿ����K�����һ��������С����K��D��ClԪ�أ�ԭ�Ӻ�����17�����ӣ����Ӿ���17���˶�״̬����5�ֲ�ͬ���ܼ���F��FeԪ�أ�ʧȥ�����������������ӣ������Ӻ�����23�����ӣ����ݹ���ԭ��֪�����Ӻ�������Ų�ʽΪ1s22s22p63s23p63d5��
��2��B���⻯���ǰ�����C���⻯�������⡢A���⻯���Ǽ��飬���⡢�����ˮ���Ӳ����γ������������ˮ�������γ����������Ĵ��ڴٽ����ܽ���������������ܽ�ȴ��ڼ�������⣻
��3��SCN���ĵ���ʽΪ��[![]() ]����
]����
��4��FeCl3��KSCN��Һ��ϵõ�������������Ѫ��ɫ��Һ�����ɵ������Ϊ���軯�أ�������λ��Ϊ5�������Ļ�ѧʽ��K2Fe��SCN��5��
��5����ͼ1��E+δ���������þ�����Fe2+����=8��4��1/8=4��Fe3+����=8��4��1/8=4��CN-����=12����8=24�����ݻ������и�Ԫ�ػ��ϼ۵Ĵ�����Ϊ0֪��K+����=��24��1-4��2-4��3��/1=4��
��6��Cu�Ķ��������������Ҷ�����H2N-CH2һCH2һNH2���γ�����������ͼ����C-Cԭ��֮����ڷǼ��Լ���C-N��C-H��N-Hԭ��֮����ڼ��Լ���ͭ���Ӻ͵�ԭ��֮�������λ�������Ը��������к��еĻ�ѧ����������λ�������Լ����Ǽ��Լ�����ѡabd��SCN�����е�Cԭ�۲���ӶԸ�����2�Ҳ����µ��Ӷԣ�����Cԭ���ӻ���ʽΪsp���Ҷ�����H2N-CH2һCH2һNH2����Cԭ�Ӽ۲���ӶԸ�����4�Ҳ����µ��Ӷԣ�����C���ӻ���ʽΪsp3��