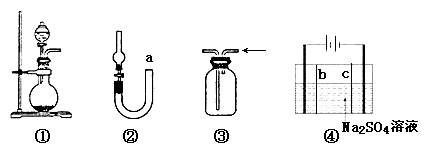
��Ŀ����
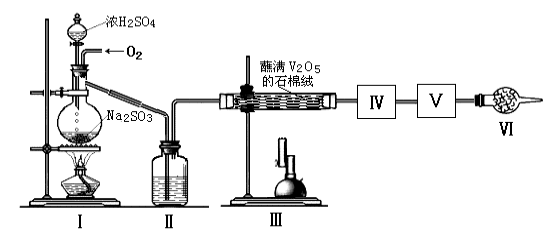
����Ŀ���ڽӴ���������������У�SO2ת����SO3ת���ʵĴ�Сֱ�Ӿ�������Ч�ʡ�ij�о�С������ͼװ��ģ�����������вⶨSO2ת����SO3��ת���ʡ���֪SO3���۵���16.8�棬�е���44.8�档����װ�������漰��Ӧ�Ļ�ѧ����ʽΪ��
Na2SO3(s)+H2SO4(75%)=Na2SO4+SO2��+H2O
��1�����е��Լ��� ��������������Ϊ ��
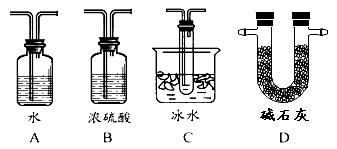
��2������ʵ����Ҫ��Ӧ���������������Ӻ��ʵ�װ�á������ͼA��Dװ����ѡ�����ʺ�װ�ò��������������Ŀո��У������������ӵ�װ�÷ֱ��� �� ��
��3��Ϊ�����SO2��ת���ʣ�ʵ��ʱ�ڣ��ٵμ�Ũ�����������ȴ����IJ����У�Ӧ��ȡ�IJ�������_______��_________(����)��
��4��ʵ�������������ռ�SO3���Թ�����¶���ڿ����У��ܹ������ܿ��д����İ����������������ԭ���� ��
��5����18.9gNa2SO3��ĩ��������Ũ��������д�ʵ�飬����Ӧ����ʱ������ͨ��O2һ��ʱ�����װ����������7.2g����ʵ����SO2��ת����Ϊ ��
���𰸡���1��Ũ���ᣨ1�֣�������ܣ�2��C��D��3���ڢ�
��4��SO3�ӷ�����������ˮ ��5��25%��3�֣�
��������
�����������1��������뷢��װ��֮ǰ��Ҫ���������Ũ������������������������������е��Լ�����Ũ������������������Ϊ�������
��2��SO3���۵���16.8�棬�����ñ�ˮ��ȴ�������������δ��Ӧ���Ķ�������Կ����������Ⱦ�������ü�ʯ�һ�������������Һ������β�����������������������ӵ�װ�÷ֱ���C��D��
��3��Ϊ��֤�����Ķ��������ܶ��ת��Ϊ��������Ӧ�ȼ��ȴ����ٵ���Ũ���
��4������SO3�ӷ�����������ˮ������ܹ������ܿ��д����İ�����
��5��18.9gNa2SO3�����ʵ�����18.9g��126g/mol��0.15mol�����ɶ���������0.15mol��װ����������7.2g����ʣ�����������7.2g�����ʵ�����7.2g��64g/mol��0.1125mol������ʵ����SO2��ת����Ϊ![]() ��100%��25%��
��100%��25%��