��Ŀ����
7���⻯�ƣ�CaH2�������ǵ�ɽ�˶�Ա���õ���Դ�ṩ�����⻯��Ҫ�ܷⱣ�棬һ���Ӵ���ˮ�ͷ�����Ӧ�����������ƺ��������⻯��ͨ��������������Ƽ�����ȡ��ͼ��ģ����ȡװ�ã�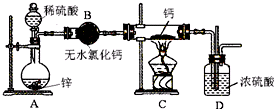
��1��װ��B�������dz�ȥ�����е�ˮ������װ��D�������Ƿ�ֹ�����е�ˮ��������Cװ�ã�
��2������ͼʵ��װ�ý���ʵ�飬ʵ�鲽�����£����װ�������Ժ�װ��ҩƷ����Һ©���������ڢ٢ܢۣ��밴��ȷ��˳���������в������ţ���
�ټ��ȷ�Ӧһ��ʱ�� ���ռ����岢�����䴿��
�۹رշ�Һ©������ ��ֹͣ���ȣ������ȴ
��3��Ϊ��ȷ�Ͻ���װ��C�������Ѿ����Ӧ��B��C֮���ٽ�һװ�ã���װ���м�����Լ��ǣ���ˮ����ͭ��
��4����ͬѧ���һ��ʵ�飬�ⶨ����ʵ���еõ����⻯�ƵĴ��ȣ������в�����Ԫ�أ�����������ʵ�鲽�裺
����Ʒ�������ڼ���Na2CO3��Һ���ѧʽ�������衢���ˣ���ϴ�ӣ���������ƣ����ܺ�ɣ���������ƣ��� �ݳ���̼��ƣ�
��5����ͬѧ����ע���������⻯�ƺ�ˮ��Ӧ��������ķ������ⶨ����ʵ���еõ����⻯�ƵĴ��ȣ�����ȡ46mg ���Ƶõ��⻯����Ʒ����¼��ʼʱע������˨ͣ����10.00mL�̶ȴ�����Ӧ����������ȴ����˨����ͣ����57.04mL�̶ȴ�����������������ڱ�״���²ⶨ������ͨ����������Ʒ���⻯�ƵĴ��ȣ�91.30%��
��6�����������һ���⻯�ƴ��ȵIJⶨ��������ȡһ��������Ʒ��m1g��������������Һ������ð���ݣ�Ȼ����Һ�����õ��Ȼ��ƹ��壨m2g��������m1��m2���ɵõ��⻯�ƵĴ��ȣ�
���� ��1���⻯��Ҫ�ܷⱣ�棬һ���Ӵ���ˮ�ͷ�����Ӧ�����������ƺ�������H2�ڷ������ȷ�Ӧ֮ǰ��Ҫ���һ������ˮ�Ȼ��ƣ���ֹ�����е�ˮ��������Cװ�ã�
��2��������μӼ��Ȼ�ȼ�յķ�Ӧ��Ҫ�����鴿��ʵ����Ϻ���Ϩ����ȴ����ֹͣ�������ɣ���ֹ����������ը��
��3�������Ƿ��������ˮ����ͭ����Ϊ��ˮ����ͭ��ˮ����ɫ��������ԣ�
��4�������ճ���̼��ƿ�֪��Ӧ����̼������Һ��ʹCaH2��Ӧ��ͬʱ�õ�̼��Ƴ�����Ȼ���ˡ�ϴ�ӡ���ɡ�������ȷ�����ȣ�
��5����ע����D��ʼʱ����ͣ����10mL�̶ȴ�����Ӧ����������ȴ����������ͣ��57.04mL�̶ȴ�����֪����������57.04mL-10mL=47.04mL����������������Ϊ��$\frac{0.04704L}{22.4L/mol}$��2g/mol=0.0042g=4.2mg�����������⻯�Ƶ�����Ϊx��������������Ϊy����Ƶ�����Ϊ46mg-x������ˮ��Ӧ������������Ϊ4.2mg-y�����ݷ���ʽCaH2+2H2O�TCa��OH��2+2H2����Ca+2H2O�TCa��OH��2+H2�����з��̼���x��y��ֵ���ٸ�����������������㣻
��6����ȡһ��������Ʒ��m1g��������������Һ������ð���ݣ���Ӧ��ȫ����Ȼ����Һ�����õ��Ȼ��ƹ��壨m2g��������m1��m2���ɵõ��⻯�ƵĴ��ȣ�
��� �⣺��1���⻯��Ҫ�ܷⱣ�棬һ���Ӵ���ˮ�ͷ�����Ӧ�����������ƺ�������H2�ڷ������ȷ�Ӧ֮ǰ��Ҫ���һ������ˮ�Ȼ��ƣ���װ��B�������ǣ���ȥ�����е�ˮ������װ��D�������ǣ���ֹ�����е�ˮ��������Cװ�ã�
�ʴ�Ϊ����ȥ�����е�ˮ��������ֹ�����е�ˮ��������Cװ�ã�
��2��������μӼ��Ȼ�ȼ�յķ�Ӧ��Ҫ�����鴿��ʵ����Ϻ���Ϩ����ȴ����ֹͣ�������ɣ���ֹ����������ը������ȷ�IJ���˳��Ϊ���ڢ٢ܢۣ�
�ʴ�Ϊ���ڢ٢ܢۣ�
��3�������Ƿ��������ˮ����ͭ����Ϊ��ˮ����ͭ��ˮ����ɫ��������ԣ�
�ʴ�Ϊ����ˮ����ͭ��
��4�������ճ���̼��ƿ�֪��Ӧ����̼������Һ��ʹCaH2��Ӧ��ͬʱ�õ�̼��Ƴ�����Ȼ���ˡ�ϴ�ӡ���ɡ�������ȷ�����ȣ�
�ʴ�Ϊ��Na2CO3��ϴ�ӡ���ɣ�
��5����ע����D��ʼʱ����ͣ����10mL�̶ȴ�����Ӧ����������ȴ����������ͣ��57.04mL�̶ȴ�����֪����������57.04mL-10mL=47.04mL����������������Ϊ��$\frac{0.04704L}{22.4L/mol}$��2g/mol=0.0042g=4.2mg�����������⻯�Ƶ�����Ϊx��������������Ϊy����Ƶ�����Ϊ46mg-x������ˮ��Ӧ������������Ϊ4.2mg-y����
CaH2+2H2O�TCa��OH��2+2H2��
42 4
x y
����42��4=x��y��������y=$\frac{2x}{21}$
Ca+2H2O�TCa��OH��2+H2��
40 2
46mg-x 4.2mg-y
����40��2=��46mg-x������4.2mg-y������y=$\frac{2x}{21}$���룬���x=42mg��������Ʒ���⻯�ƵĴ���Ϊ��$\frac{42mg}{46mg}$��100%=91.30%��
�ʴ�Ϊ��91.3%��
��6����ȡһ��������Ʒ��m1g��������������Һ������ð���ݣ���Ӧ��ȫ����Ȼ����Һ�����õ��Ȼ��ƹ��壨m2g��������m1��m2���ɵõ��⻯�ƵĴ��ȣ�
�ʴ�Ϊ����ȡһ��������Ʒ��m1g��������������Һ������ð���ݣ���Ӧ��ȫ����Ȼ����Һ�����õ��Ȼ��ƹ��壨m2g��������m1��m2���ɵõ��⻯�ƵĴ��ȣ�
���� �������⻯���Ʊ�Ϊ���壬����ʵ�����������������Ժ����е���Ϣ�����á���ʵ��װ�õ�������������ʷ����ᴿ����ѧ���㡢ʵ�鷽����Ƶȣ�������ػ���ʵ������������飬�Ƕ�ѧ���ۺ������Ŀ��飬�Ѷ��еȣ�

 Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д�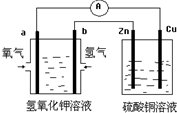
| A�� | ��װ����Cu��Ϊ���� | |
| B�� | ����ʱ�������K+����a�缫 | |
| C�� | ����һ��ʱ�䣬Ҫʹ�ҳ���Һ��ԭ�ɼ���������CuO | |
| D�� | b���ĵ缫��Ӧʽ��H2+2OH--2e-�T2H2O |
��ˮ���Ƶõ��Ȼ��Ƴ�ʳ���⣬��������ҵԭ�ϣ�����ͨ����NaCI��CO2��NH3Ϊԭ����ȡ�����д����һ����ȡNaHCO3�Ļ�ѧ����ʽ��NH3+CO2+H2O+NaCl�TNaHCO3��+NH4Cl��
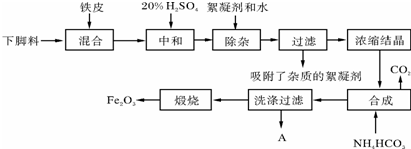
��ҵ����Ũ����ˮΪԭ����ȡ��IJ���������ͼ1��
��֪��Br2�����³�Һ̬���ӷ����ж���2Br2+3CO32-=5Br-+BrO3-+3CO2��
��1��ͨ����������������ѻ�ú�Br2����Һ��Ϊ�λ��辭�����������ա��ữ�����»�ú�Br2����Һ��ԭ���Ǹ����壬���Br2��Ũ�ȣ�
��2����Ӧ��2�з�����Ӧ�����ӷ���ʽΪ5Br-+BrO3-+6H+=3Br2+3H2O��
��3������������ͨ��ˮ�������ȣ������¶���90�����ҽ��������ԭ�����¶ȹ������Խ�Br2�������������¶ȹ����ֻὫ������ˮ���������
����±������ȴ������±�����Ҫ�ɷ���MgCI2���������Fe2+��Fe3+��Mn2+�����ӣ���±��Ϊԭ���Ƶ�þ�Ĺ���������ͼ2�����ֲ�������������ȥ����
�����������������pH
| ���� | ��ʼ���� | ������ȫ |
| Fe��OH��3 | 2.7 | 3.7 |
| Fe��OH��2 | 7.6 | 9.6 |
| Mn��OH��2 | 8.3 | 9.8 |
| Mg��OH��2 | 9.6 | 11.1 |
��2�����������HCI�������м��Ƚ��У����û�ѧƽ���ƶ�ԭ������ԭ��Mg2++2H2O
 Mg��OH��2+2H+���¶����ߣ�ˮ��̶�����ͨ��HCl������c��H+����ʹƽ�������ƶ�������Mg2+ˮ�⣮HCl���ܴ���ˮ�ݣ����յõ���ˮMgCl2��
Mg��OH��2+2H+���¶����ߣ�ˮ��̶�����ͨ��HCl������c��H+����ʹƽ�������ƶ�������Mg2+ˮ�⣮HCl���ܴ���ˮ�ݣ����յõ���ˮMgCl2����3��NaCIO���ܳ�ȥ��±�е�CO��NH2��2�������κ��ܲ������ѭ�������ʣ���ȥ0.1molCO��NH2��2ʱ����NaCIO22.35g��

| A�� | ԭ�ӵ�������������ͬ��Ԫ�أ�һ������ͬһ�� | |
| B�� | ���Ӳ�����ͬ�����ӣ���ӦԪ��һ������ͬһ���� | |
| C�� | Ԫ�����ڱ���Ԫ�������������ԭ�ӵĺ˵���� | |
| D�� | Ԫ�����ڱ�����ʮ�������У�Ҳ����ʮ������ |
| A�� | ��Ϊ����HF��HCl�����Էǽ�����F��Cl | |
| B�� | ��ΪSO2��Cl2����Ư���ԣ����Զ����ʹ��Ư����ɫ���ʣ�Ч������� | |
| C�� | ��Ϊij���ʵ���ɫ��Ӧ�ʻ�ɫ��������һ���Ǻ�NaԪ�صĻ����� | |
| D�� | ��Ϊϩ����ͨʽΪCnH2n����ͬϩ����ʵ��ʽ��ͬ������ϩ���ĺ�̼��Ϊһ���� |