��Ŀ����
17���±��г��ˢ١�������Ԫ�������ڱ��е�λ�ã�
�밴Ҫ��ش��������⣮
��1��Ԫ�آ������ڱ��е�λ���ǵڶ����ڣ��ڢ�A�壮
��2���١��ݡ�������Ԫ�ص�����������Ӧ��ˮ�����У�������ǿ����NaOH���ѧʽ����
��3��Ԫ�آ����ڷǽ������������ǽ�����Ԫ�أ�������������ϼ���+5�����ĵ�����H2��һ�������·�Ӧ���ɻ�����X��X��ˮ��Һ�Լ��ԣ������ԣ����Ի����ԣ���
��4��Ԫ�آ���Ԫ�آ��γɵĻ������л�ѧ�������������Ӽ���
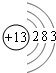
��5������Ԫ�آ�ԭ�ӽṹʾ��ͼ
 �����ĵ����ܣ����ܻ��ܣ�������������Һ��Ӧ��
�����ĵ����ܣ����ܻ��ܣ�������������Һ��Ӧ����6��Ԫ�آ��γɵ�����л���ķ���ʽΪCH4��
���� ��Ԫ�������ڱ��е�λ�ÿ�֪������Li������C������N������F������Na������Al������Cl��
��1����Ԫ�ص�λ�ÿ�֪�ܴ��ڵڶ����ڢ�A�壻
��2��������Խǿ������������Ӧˮ����ļ���Խǿ��
��3������NԪ�أ����ڷǽ���Ԫ�أ���������ϼ۵�����������������������Ӧ���ɰ�������ˮ��һˮ�ϰ������笠����������������ӣ���Һ�ʼ��ԣ�
��4��Ԫ�آ���Ԫ�آ��γɵĻ�����ΪNaCl��
��5������Al��ԭ�Ӻ�����13�����ӣ���3�����Ӳ㣬���������Ϊ2��8��3��Al������������Һ��Ӧ����ƫ��������������
��6��Ԫ�آ��γɵ�����л���Ϊ���飮
��� �⣺��Ԫ�������ڱ��е�λ�ÿ�֪������Li������C������N������F������Na������Al������Cl��
��1����Ԫ�ص�λ�ÿ�֪�ܴ��ڵڶ����ڢ�A�壬�ʴ�Ϊ��������A��
��2��Li��Na��Al�У�Na�Ľ�����Խǿ��������������Ӧˮ������NaOH�ļ�����ǿ���ʴ�Ϊ��NaOH��
��3������NԪ�أ����ڷǽ���Ԫ�أ���������ϼ۵�����������Ϊ+5��������������Ӧ���ɰ�������ˮ��һˮ�ϰ������笠����������������ӣ���Һ�ʼ��ԣ�
�ʴ�Ϊ���ǽ�����+5�����ԣ�
��4��Ԫ�آ���Ԫ�آ��γɵĻ�����ΪNaCl���������Ӽ����ʴ�Ϊ�����Ӽ���
��5������Al��ԭ�Ӻ�����13�����ӣ���3�����Ӳ㣬���������Ϊ2��8��3��ԭ�ӽṹʾ��ͼΪ ��Al������������Һ��Ӧ����ƫ��������������
��Al������������Һ��Ӧ����ƫ��������������
�ʴ�Ϊ�� ���ܣ�
���ܣ�
��6��Ԫ�آ��γɵ�����л���ΪCH4���ʴ�Ϊ��CH4��
���� ���⿼��Ԫ�����ڱ���Ԫ���������ۺ�Ӧ�ã���Ҫѧ����������Ԫ�����ڱ����ѶȲ��������ڻ���֪ʶ�Ĺ��̣�

 �ǻۿ����ܾ�100�ֵ�Ԫ���ؼ��ϵ�д�
�ǻۿ����ܾ�100�ֵ�Ԫ���ؼ��ϵ�д� ��Ԫ������ĩ��ϵ�д�
��Ԫ������ĩ��ϵ�д�| A�� | ��ˮ | B�� | ����ˮ | C�� | �� | D�� | CCl4 |
| A�� | ÿ����l mol CH4���������·�ṩ8 mol e- | |
| B�� | ������CH4ʧȥ���ӣ��缫��ӦʽΪCH4+10OH--8e-�TCO32-+7H2O | |
| C�� | ��طŵ�ʱ������������pH���Ͻ��� | |
| D�� | ��طŵ�ʱ����Һ�е�OH-�������ƶ� |
| A�� | 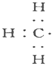 --���ĵ���ʽ --���ĵ���ʽ | B�� | ����Ľṹʽ��CH4 | ||
| C�� | CH3-CH=CH-CH3�ļ���ʽ�� | D�� | ���ķ���ʽ�� |
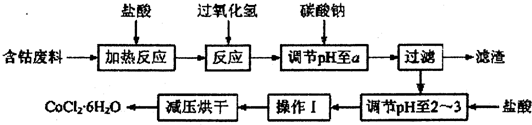
��֪��
��CoCl2•6H2O�۵�86�棬������ˮ�����ѵȣ��������ȶ�����������110��120��ʱ��ʧȥ�ᾧˮ����ж�����ˮ�Ȼ��ܣ�
�ڲ���������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe��OH��3 | Fe��OH��2 | Co��OH��2 | Al��OH��3 |
| ��ʼ���� | 2.3 | 7.5 | 7.6 | 3.4 |
| ��ȫ���� | 4.1 | 9.7 | 9.2 | 5.2 |
��1���������ᷴӦ�Ļ�ѧ����ʽΪCo+2HCl=CoCl2+H2����
��2�������м���̼���Ƶ���pH��a��a�ķ�Χ��5.2��7.6�������к��е�Al��OH��3�����õ���ȼ������ԭ����Al��OH��3���ȷֽ�ʱ���մ������ȣ�ʹ�����¶��½���ͬʱ���ɵ����¡��ȶ��Ժõ�Al2O3�����ڿ�ȼ����棬��ȼЧ�����ѣ����������pH��2��3��Ŀ��������Co2+��ˮ�⣬��ֹ�ں����IJ������γ�Co��OH��2���ʣ�
��3�����������3������ʵ�����������������Ũ������ȴ�ᾧ���ˣ�
��4���Ƶõ�CoCl2•6H2O���ѹ��ɵ�ԭ���ǽ��ͺ���¶ȣ���ֹ��Ʒ�ֽ⣮
��5��Ϊ�ⶨ��Ʒ��CoCl2•6H2O������ijͬѧ��119g��Ʒ����ˮ�γ�100ml��Һ��ȡ25mL���ձ��м���������AgNO3��Һ�����ˣ�����������ɺ�Ƶ�����Ϊ28.7g�������Ʒ��CoCl2•6H2O����Ϊ80%������֪��CoCl2•6H2O��ѧʽ��Ϊ238��AgClΪ143.5���������ʲ���AgNO3��Һ��Ӧ�����������λ��Ч���֣�
| A�� | C3H7OH | B�� | 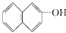 | C�� | CH2OHCHOHCH2OH | D�� | 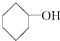 |
| A�� | ���մɲ�������п | B�� | SO2��O2��Ӧ�Ĵ������������� | ||
| C�� | �뵼������黯�� | D�� | ������������Ͻ� |
 ����
���� �����ַ�Ӧ���Ƶõģ������ַ�Ӧ�����ǣ�������
�����ַ�Ӧ���Ƶõģ������ַ�Ӧ�����ǣ�������| A�� | ��ȥ���Ӿ� | B�� | ˮ�⡢���� | C�� | ���������� | D�� | ȡ�����Ӿ� |