��Ŀ����
��ѧ�������֮����ת�����˵�����ʵ��������أ�������������������Ҫ��Ӧ�ã�ͬʱҲ��ѧ���γɻ�ѧѧ����������Ҫ��ɲ��֡�
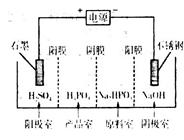
��1������״̬�£��Ƶĵ��ʺ��Ȼ���������ɿɳ����(��ͼ1)����Ӧԭ��Ϊ��2Na��FeCl2  Fe��2NaCl,�õ�طŵ�ʱ��������ӦʽΪ ________________ _____��
Fe��2NaCl,�õ�طŵ�ʱ��������ӦʽΪ ________________ _____��
���ʱ��__________(д��������)�缫�ӵ�Դ�ĸ�����
�õ�صĵ����Ϊ________ _��
��2��ijͬѧ��ͭƬ��ʯī���缫���һ��Ũ�ȵ�����ͭ��Һ(��ͼ2)��һ��ʱ��ֹͣͨ��ȡ���缫�����ڵ������Һ�м���0.98g������ͭ��ĩǡ����ȫ�ܽ⣬���ⶨ������Һ����ǰ��ȫ��ͬ����ش��������⣺
��Y�缫������ ������ (�������ԭ��)��Ӧ��
�ڵ�������X�缫�Ϸ����ĵ缫����Ӧʽ�ǣ�
�����ڵ������Һ�м���������С�մ�ַ�Ӧ����������ڱ�״������ռ�������
��3������ʱ��BaSO4��Ksp��1.08��10-10,�ֽ��������BaCl2��Һ��2.0��10-3mol/l��Na2SO4
��Һ��ϡ���Ҫ����BaSO4������BaCl2��Һ����СŨ��Ϊ______________��
(14��)
��1��Fe2��+2e��= Fe��2�֣����ƣ�1�֣����¡�Al2O3��1�֣�
��2����ʯī��1�֣���������1�֣�
��Cu2����2e��=Cu��2�֣���2H����2e��=H2����2H2O��2e��=H2����2OH����2�֣�
��0.448L��448mL��2�֣�û�е�λ��1��
��3��2.16x10-7. ��2�֣�
������������� ��1����������ԭ��Ӧ�������ϼ۽��͵�Ԫ�ط�����ԭ��Ӧ�����������ʱ���������������ܵ�������������Һ��
��2���ɼ���0.98g������ͭ����ȫ�ܽ⣬������Һ����ǰ��ȫ��ͬ����֪��Һ���������ͭ���������ŵ����Cu2�����������ŵ����OH-��С�մ�NaHCO3��
Cu SO4 + 2 H2O= Cu���ϣȣ�����H2SO4��n=0.98g/98gmol-1=0.01mol
��NaHCO3��H2SO4��Na��SO4��H2O����CO������������
0.01mol 0.02mol
0.02mol��22.4L/mol=0.448L
��3�� �������BaCl2��Һ��2.0��10-3mol/l��Na2SO4��Һ���
Ksp���㣨Ba2�����㣨SO4���������㣨Ba2������.0��10-3��1.08��10-10
�㣨Ba2������1.08��10-7����ԭ��Ba2����=2.16x10-7.
���㣺���⿼�����֪ʶ��Ksp�ļ����֪ʶ��

 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д� ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д� ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д���1��25��ʱ��ijNaCl��Һ��c(Cl�C)��1��10��4 mol��L�C1�������Һ��c(Na��)��c(OH��)��
��2��25��ʱ����0.1 mol��L�C1NaOH��Һ��0.06 mol��L�C1��H2SO4��Һ��������(���Ի�Ϻ�����ı仯)����������Һ��pH�� ��25��ʱ��pHֵΪ8��NaOH��Һ��pHֵΪ10��NaOH��Һ�������Ϻ���Һ��������Ũ����ӽ� ��
��3��25��ʱ������������Һ�У���pH=0������ ��0.1 mol��L�C1������ ��0.01 mol��L�C1��NaOH��Һ ��pH=11��NaOH��Һ����ˮ��������������Ũ��֮�Ȣ٩U�کU�۩U���ǣ� (����ĸ)
| A��1�U10�U100�U1000 | B��0�U1�U12�U11 |
| C��14�U13�U12�U11 | D��14�U13�U2�U3 |
������¶���ˮ��Kw�� ��
���ڸ��¶��²��ij��ҺpH��3�������Һ��c(H��)��c(OH��)��________��
�۸��¶��½�pH=2�������pH=11������������Һ�������ϣ�pH=______________
��5�� ��ˮ��c(H+)=5.0��10�C7 mol��L�C1�����ʱ��ˮ�е�c(OH�C) = �����¶Ȳ��䣬����ϡ����ʹc(H+)=5.0��10�C3 mol��L�C1����c(OH�C) = ���ڸ��¶�ʱ����ˮ�е���NaOH��Һ����Һ�е�c(OH�C)=5.0��10�C2 mol��L�C1������Һ��c(H+)= ��
ijʳ�ð״����ɴ����봿ˮ���ƶ��ɣ����к͵ζ��ķ���ȷ�ⶨ���д�������ʵ���Ũ�ȡ�ʵ�鲽�裺������500mLŨ��ԼΪ0��1mol��L-1��NaOH��Һ������KHC8H4O4����Һȷ�ⶨ��NaOH��Һ��Ũ�ȣ�������֪ȷŨ�ȵ�NaOH��Һ�ⶨ�����Ũ�ȡ�
��1�����������NaOH�������ڴ��ձ��У�����500mL����ˮ�������ܽ⡣�����Ʋ��� ������С������С�����
��2������ʱNaOH�ڿ����м�����ˮ���������õ�NaOH��ҺŨ��ͨ����Ԥ�� ���С���������Dz���ֱ�����������Һ��ԭ��
��3�����İ״װ�װ�����Ậ��ԼΪ6g/100mL����������ʵ�Ũ��ԼΪ mol��L-1���ζ�ǰҪ�Ƚ��״�ϡ��10����ϡ�Ͱ״�ʱ��Ҫ��������100mL����ƿ���ձ����������� ��ͷ�ιܡ� ��
��4��ȷ��ȡϡ�ͺ�İ״�20��00mL������250mL��ƿ�У�����30mL����ˮ���ٵμӷ�ָ̪ʾ����������NaOH����Һ�ζ��� ��Ϊ�յ㡣
��5��Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ����ȡ�״������Ϊ20��00mL��NaOH��ҺŨ��Ϊc mo1/L������ʵ ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 25��02 | 24��22 | 24��18 |
���ϱ����Կ�������һ��ʵ���м�¼����NaOH��Һ��������Զ��ں����Σ���ԭ������� ��
A���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������
B��ʢװ��Һ�ĵζ���װҺǰ������ˮ��ϴ����δ�ñ�Һ��ϴ
C����һ�εζ��õ���ƿδ��ϴ
D���ζ�����ʱ�����Ӷ���
��6�������������ݣ�д������ð״��д�������ʵ���Ũ�ȣ� ��
�������ƣ�NaNO2������Ҫ�ķ�������ij��ѧ��ȤС�鳢���Ʊ��������ƣ��������ϣ���HNO2Ϊ���ᣬ��������Һ�У�NO2-�ɽ�MnO4-��ԭΪMn2�������������ɡ�
��NO����Ӧ���ɱ�����KMnO4��Һ����Ϊ����
̽��һ �������ƹ�����Ʊ�
��̼��Ũ����Ϊ��ʼԭ�ϣ��������װ������һ��������������Ʒ�Ӧ�Ʊ��������ơ�����Ӧ����ʽΪ2NO��Na2O2=2NaNO2�����ּг�װ�ú�A�м���װ�����ԣ�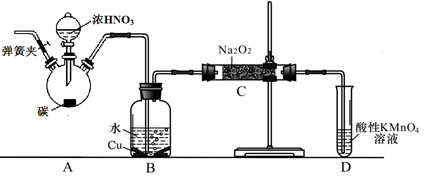
��1��д��װ��A��ƿ�з�����Ӧ�Ļ�ѧ����ʽ ��
��2����ͬѧ��Ϊװ��C�в��ﲻ�����������ƣ�����̼���ƺ��������ƣ�Ϊ�ų�����Ӧ��B��Cװ�ü�����װ��E��E��ʢ�ŵ��Լ�Ӧ�� ������ĸ����
A��ŨH2SO4 B����ʯ�� C����ˮCaCl2
̽���� �������ƹ��庬���IJⶨ��������֤
��ȡװ��C�з�Ӧ��Ĺ���4.000g����ˮ���250mL��Һ��ȡ25.00mL��Һ����ƿ�У���0.1000
mol/L����KMnO4��Һ���еζ���ʵ�������������±���ʾ��
| ����� | 1 | 2 | 3 | 4 |
| KMnO4��Һ���/mL | 20.60 | 20.02 | 20.00 | 19.98 |
��3����һ��ʵ�����ݳ����쳣����������쳣��ԭ������� ������ĸ����
A����ʽ�ζ���������ˮϴ����δ�ñ�Һ��ϴ
B����ƿϴ����δ����
C���ζ��������Ӷ���
��4�����ݱ������ݣ��������ù������������Ƶ��������� ��
��5����������������ˮ����0.2mol��L-1������������Һ��0.1mol��L-1������������ϣ���Ϻ���Һ�����ԣ����Ϻ���Һ������Ũ���ɴ�С��˳��Ϊ ��
̽���� ��Ӧ��Һ�Ĵ���
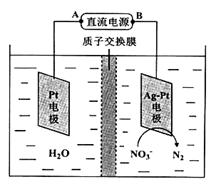
��Ӧ����ƿA����Ȼ����һ���������ᣬ����ֱ���ŷţ���NaOH��Һ�������ԣ����õ绯ѧ���ⷨ���д�����25��ʱ����Ӧ����10min����Һ��pH��7��Ϊ12���绯ѧ����NO3-��ԭ������ͼ��ʾ��
��6����Դ����Ϊ ����A��B����������ӦʽΪ ��
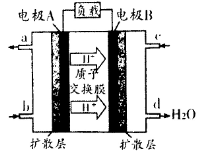
����ͼ��ʾ����пƬ��ͭƬ�õ������������װ��ϡ������Һ���ձ��й���ԭ��ء�����������ȷ����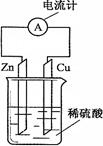
| A��Zn�Ǹ�����������ԭ��Ӧ |
| B��������пƬ����ͭƬ |
| C��һ��ʱ���ͭƬ�������� |
| D����װ�ý���ѧ��ת��Ϊ���� |
 2G��g��������
2G��g��������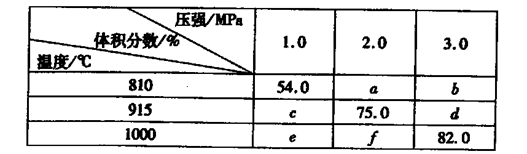
 ����Cr3��Ũ��С��10
����Cr3��Ũ��С��10 mol
mol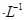 ʱ����Ϊ��ȫ�����������ȫ�����Һ��pH=6�������Һ���˺�Ϊ___________����ܡ���ֱ���ŷš�
ʱ����Ϊ��ȫ�����������ȫ�����Һ��pH=6�������Һ���˺�Ϊ___________����ܡ���ֱ���ŷš� ________���������С�����䡱����25
________���������С�����䡱����25 ʱ��NH3?H2O�ĵ���ƽ�ⳣ��
ʱ��NH3?H2O�ĵ���ƽ�ⳣ��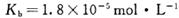 �����¶���,1mol
�����¶���,1mol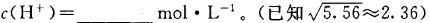
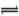 X2��+H+���ش��������⣺
X2��+H+���ش��������⣺ ��2����25���£���Ũ�Ⱦ�Ϊ0.1 mol��L��1��FeCl3��AlCl3�����Һ����μ��백ˮ�������� ���ѧʽ����������֪25��ʱKsp[Fe(OH)3]=2.6��10��39 mol4��L��4��KsP[Al(OH)3]=1.3��10��33 mol4��L��4��
��2����25���£���Ũ�Ⱦ�Ϊ0.1 mol��L��1��FeCl3��AlCl3�����Һ����μ��백ˮ�������� ���ѧʽ����������֪25��ʱKsp[Fe(OH)3]=2.6��10��39 mol4��L��4��KsP[Al(OH)3]=1.3��10��33 mol4��L��4��