��Ŀ����
����Cu2O���ھ��������Ĵ����ܶ��ܵ���ע���±�Ϊ��ȡCu2O�����ַ�����
��1����ҵ�ϳ��÷�����ͷ�������ȡCu2O�������÷�������ԭ���Ƿ�Ӧ���������ƣ������²��������� ��ʹCu2O���ʽ��͡�
��2����֪��2Cu(s)��1/2O2(g)=Cu2O(s) ��H =-akJ��mol-1
C(s)��1/2O2(g)=CO(g) ��H =-bkJ��mol-1
Cu(s)��1/2O2(g)=CuO(s) ��H =-ckJ��mol-1
�������ķ�Ӧ��2CuO(s)��C(s)= Cu2O(s)��CO(g)����H = kJ��mol-1��
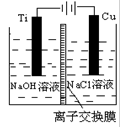
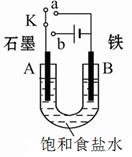
��3��������������ӽ���Ĥ���Ƶ��Һ��OH-��Ũ�ȶ��Ʊ�����Cu2O��װ����ͼ��ʾ���õ�ص���������Cu2O��ӦʽΪ ��
��4��������Ϊ������������Һ̬�£�N2H4����ԭ����Cu(OH)2���Ʊ�����Cu2O��ͬʱ�ų�N2�����Ʒ��Ļ�ѧ����ʽΪ ��
��5������ͬ���ܱ������У����������ַ����Ƶõ�Cu2O�ֱ���д��ֽ�ˮ��ʵ�飺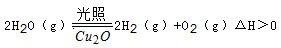
ˮ������Ũ�ȣ�mol/L����ʱ��t(min)�仯���±���ʾ��
����������ȷ���� ������ĸ���ţ���
A��ʵ����¶�T2С��T1
B��ʵ���ǰ20 min��ƽ����Ӧ����v(O2)=7��10-5 mol��L-1 min-1
C��ʵ��ڱ�ʵ������õĴ�����Ч�ʸ�
| ������ | ��̿���ڸ��������»�ԭCuO |
| ������ | ��ⷨ����ӦΪ2Cu + H2O  Cu2O + H2���� Cu2O + H2���� |
| ������ | ���£�N2H4����ԭ����Cu(OH)2 |
��1����ҵ�ϳ��÷�����ͷ�������ȡCu2O�������÷�������ԭ���Ƿ�Ӧ���������ƣ������²��������� ��ʹCu2O���ʽ��͡�
��2����֪��2Cu(s)��1/2O2(g)=Cu2O(s) ��H =-akJ��mol-1
C(s)��1/2O2(g)=CO(g) ��H =-bkJ��mol-1
Cu(s)��1/2O2(g)=CuO(s) ��H =-ckJ��mol-1
�������ķ�Ӧ��2CuO(s)��C(s)= Cu2O(s)��CO(g)����H = kJ��mol-1��
��3��������������ӽ���Ĥ���Ƶ��Һ��OH-��Ũ�ȶ��Ʊ�����Cu2O��װ����ͼ��ʾ���õ�ص���������Cu2O��ӦʽΪ ��

��4��������Ϊ������������Һ̬�£�N2H4����ԭ����Cu(OH)2���Ʊ�����Cu2O��ͬʱ�ų�N2�����Ʒ��Ļ�ѧ����ʽΪ ��
��5������ͬ���ܱ������У����������ַ����Ƶõ�Cu2O�ֱ���д��ֽ�ˮ��ʵ�飺
ˮ������Ũ�ȣ�mol/L����ʱ��t(min)�仯���±���ʾ��
| ��� | �¶� | 0 | 10 | 20 | 30 | 40 | 50 |
| �� | T1 | 0.050 | 0.0492 | 0.0486 | 0.0482 | 0.0480 | 0.0480 |
| �� | T1 | 0.050 | 0.0488 | 0.0484 | 0.0480 | 0.0480 | 0.0480 |
| �� | T2 | 0.10 | 0.094 | 0.090 | 0.090 | 0.090 | 0.090 |
A��ʵ����¶�T2С��T1
B��ʵ���ǰ20 min��ƽ����Ӧ����v(O2)=7��10-5 mol��L-1 min-1
C��ʵ��ڱ�ʵ������õĴ�����Ч�ʸ�
��1��ͭ����Cu��
��2��-��a+b-2c��kJ/mol����2c�Ca-b��
��3��2Cu��2e����2OH��=Cu2O��H2O
��4��4Cu(OH)2 + N2H4 2Cu2O + N2�� + 6H2O
2Cu2O + N2�� + 6H2O
��5��C
��2��-��a+b-2c��kJ/mol����2c�Ca-b��
��3��2Cu��2e����2OH��=Cu2O��H2O
��4��4Cu(OH)2 + N2H4
 2Cu2O + N2�� + 6H2O
2Cu2O + N2�� + 6H2O ��5��C
��1����̿���ڸ��������»�ԭCuO�������²���������ͭ��ʹCu2O���ʽ��ͣ�
��2�����ݸ�˹���ɣ�����������ʽ���ηֱ�ΪA��B��C���������Ȼ�ѧ��ʽΪ
A+B-2C����2CuO��s��+C��s��=Cu2O��s��+CO��g����H=-(a+b-2c)kJ/mol��
��3�������缫��Ӧʽ�ǣ�2Cu��2e����2OH��=Cu2O��H2O��
��4��N2H4��Cu(OH)2��Ӧ�������Cu2O��N2���ˮ���ɣ��䷽��ʽΪ4Cu(OH)2+ N2H4 2Cu2O + N2�� + 6H2O
2Cu2O + N2�� + 6H2O
��5��ʵ��ڢ���ȣ�ʵ���ˮ��������ʼ���ʵ���Ũ��ʵ��ڵ�2������ƽ�����ʵ���Ũ��ȴС��2����˵��T1��T2ƽ�����������ƶ���������ӦΪ���ȣ�����TI��T2�������¶ȣ�T2����T1��A�������ݷ�Ӧ���ʵĶ��壬ʵ���ǰ20min��ƽ����Ӧ����V��H2O��=7��10-5mol/L������v(O2)=3.5��10-5mol��L-1 min-1��B����ʵ��ں͢���ȣ��ﵽƽ��״̬��ͬ��������ʱ��̣���Ӧ���ʿ죬����ʵ��ڱ�ʵ������õĴ���Ч�ʸߣ���ȷ��
��2�����ݸ�˹���ɣ�����������ʽ���ηֱ�ΪA��B��C���������Ȼ�ѧ��ʽΪ
A+B-2C����2CuO��s��+C��s��=Cu2O��s��+CO��g����H=-(a+b-2c)kJ/mol��
��3�������缫��Ӧʽ�ǣ�2Cu��2e����2OH��=Cu2O��H2O��
��4��N2H4��Cu(OH)2��Ӧ�������Cu2O��N2���ˮ���ɣ��䷽��ʽΪ4Cu(OH)2+ N2H4
 2Cu2O + N2�� + 6H2O
2Cu2O + N2�� + 6H2O ��5��ʵ��ڢ���ȣ�ʵ���ˮ��������ʼ���ʵ���Ũ��ʵ��ڵ�2������ƽ�����ʵ���Ũ��ȴС��2����˵��T1��T2ƽ�����������ƶ���������ӦΪ���ȣ�����TI��T2�������¶ȣ�T2����T1��A�������ݷ�Ӧ���ʵĶ��壬ʵ���ǰ20min��ƽ����Ӧ����V��H2O��=7��10-5mol/L������v(O2)=3.5��10-5mol��L-1 min-1��B����ʵ��ں͢���ȣ��ﵽƽ��״̬��ͬ��������ʱ��̣���Ӧ���ʿ죬����ʵ��ڱ�ʵ������õĴ���Ч�ʸߣ���ȷ��

��ϰ��ϵ�д�
�����Ŀ

 2OH�D��H2��
2OH�D��H2��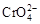 ��2H��
��2H��
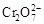 ��H2O�����ƶ�
��H2O�����ƶ� 2K2Cr2O7��4KOH��2H2����2O2��
2K2Cr2O7��4KOH��2H2����2O2��
 Cu2O+H2��
Cu2O+H2��
 Al2O3 + 3H2�����������У������ж���ȷ���ǣ�������
Al2O3 + 3H2�����������У������ж���ȷ���ǣ�������