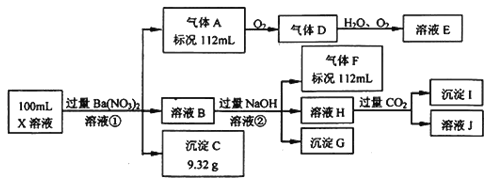
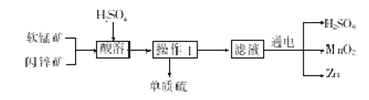
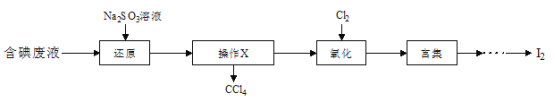
��Ŀ����
����Ŀ������̼(COS)����������������Ѭ������
��1���������̼������ĸ�ԭ���У�ԭ�Ӱ뾶����Ԫ�������ڱ��е�λ����_______________.
��2��������ʵ�����ڱȽ�C��P����Ԫ�طǽ��������ǿ������_____(����ĸ)
A����������ϼۣ�P��C
B��ͬ��ͬŨ�ȵ�����Һ�����ԣ�H3PO4��H2CO3
C���е㣺PH3��CH4
��3������̼ˮ�⼰����Ӧ���������£����ֲ�������ȥ����
![]()
����֪�������£���Ӧ����ÿ����1.7g H2S���壬��Ӧ�ų�����4.76kJ����÷�Ӧ���Ȼ�ѧ����ʽΪ_______________________________��
����֪M��Һ����Ԫ�ص���Ҫ������ʽΪS2O32������Ӧ��������S2O32�������ӷ���ʽΪ_______________.
����ͼ�Ƿ�Ӧ���У��ڲ�ͬ��Ӧ�¶��£���Ӧʱ����H2�����Ĺ�ϵ��Na2S��ʼ����Ϊ3mmol������ͼ�������֪��a��ʱM��Һ�г�S2O32���⣬����______________(����������ӷ���)��

���𰸡���1����������VA����1������
��2��b��2������
��3����H2S��g��+2NaOH��aq��==Na2S��aq��+2H2O��l�� ��H=-95.2kJ��mol-1��
��2S2-+5H2O![]() S2O32-+4H2��+2OH-��
S2O32-+4H2��+2OH-��
��SO42-��������������������2������
��������
�����������1���������̼�������ԭ��ΪO��S��P��H����Ԫ����1�����Ӳ�����Ԫ����2�����Ӳ����ס�����3�����Ӳ��������ס����ԭ�Ӱ뾶�����⡢����ԭ�Ӱ뾶���ס�����ͬ����Ԫ�������ԭ������������ԭ����������Ԫ��������֪����ԭ�Ӱ뾶�������ԭ�Ӱ뾶���������Ϊ5�����������ڵ������ڵ�VA������2���Ƚ�����Ԫ�صķǽ�����ǿ�����ɸ��ݵ���֮����û���Ӧ����������Ӧ�����׳̶ȡ��⻯����ȶ����Լ���������������Ӧˮ��������ǿ���ȽǶ�������������ϼۡ��⻯��ķе�ߵͲ������ڱȽ�Ԫ�صķǽ�����ǿ������ѡ��b������������3������Ӧ��Ϊ������������Ƶķ�ӦH2S+2NaOH=Na2S+H2O��1.7gH2S�����ʵ���Ϊn(H2S)=1.7g��34g/mol=0.05mol����Ӧ�ų�����4.76kJ����1mol���ⷴӦ�ų�95.2KJ�����������Ը÷�Ӧ���Ȼ�ѧ����ʽΪH2S��g��+2NaOH��aq��==Na2S��aq��+2H2O��l�� ��H=-95.2kJ��mol-1�������������Ϣ��S2-��ˮ��Ӧ����S2O32-��H2��������ԭ���غ��������غ㡢����غ����ɵ÷�Ӧ�����ӷ���ʽ����2S2-+5H2O![]() S2O32-+4H2��+2OH-������Ӧ������a��360��ʱ������ʱ����������������������Na2S��ʼ����Ϊ3mmol����ֻ������2S2-+5H2O
S2O32-+4H2��+2OH-������Ӧ������a��360��ʱ������ʱ����������������������Na2S��ʼ����Ϊ3mmol����ֻ������2S2-+5H2O![]() S2O32-+4H2��+2OH-������������3mmol��4/2=6mmol��ͼ��Ϊ9mmol��˵��M��Һ�г�S2O32-��������SO42-����Ӧ����ʽΪ��S2-+4H2O
S2O32-+4H2��+2OH-������������3mmol��4/2=6mmol��ͼ��Ϊ9mmol��˵��M��Һ�г�S2O32-��������SO42-����Ӧ����ʽΪ��S2-+4H2O![]() SO42-+4H2����
SO42-+4H2����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�