��Ŀ����
����Ŀ��ijǿ������ҺX�н�����Ba2+��Al3+��NH4+��Fe2+��Fe3+��CO32����SO32����SO42����Cl����NO3���е�һ�ֻ��֣�ȡ����Һ��������ʵ�飬ʵ��������£�
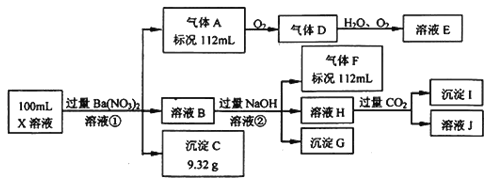
����������Ϣ���ش��������⣺
��1����������������ʵ�鲻��ȷ����ҺX���Ƿ��е����������ӷֱ��� ����Ҫ��ʵ��֤�����������Ƿ���ڣ���ɿ��Ļ�ѧ������ ��
��2������F�������������̣�д���˷�Ӧ�Ļ�ѧ����ʽ ��
��3��������������������Ũ��Ϊ2mol/L��������l0mLʱ��ʼ����������55mLʱ���������ﵽ���ֵ0.03mol�������μӳ����������ֲ��䣬����ٵμӳ��������ܽ⣬��60mLʱ����������Ϊ0.025mol�ұ��ֲ��䣬��ԭ��Һ��c��Fe2����Ϊ mol/L��c��Fe3+��Ϊ mol/L��c��Cl����Ϊ mol/L��������Щ���Ӳ����ڣ�����0mol/L��
���𰸡���1��Fe3+��Cl����ȡ����B��Һ���Թ����������еμ�AgNO3��Һ�����а�ɫ����������˵������Cl��������Cl��������
��2��8NH3+3Cl2=6 NH4Cl+N2
��3��0.15��0.1��0.4
��������
���������ǿ������Һ��һ���������CO32-��SO32-���ӣ�����������ᱵ���ɳ�������ó���C9.32gΪBaSO4��˵����Һ�к���SO42-���ӣ��������ᱵ���������Լ�����������ӵ����ʵ���Ϊ![]() =0.04mol��Ũ����0.04mol/0.1L=0.4mol/L����������A��A������������D��E����AΪNO��DΪNO2��EΪHNO3��˵����Һ�к��л�ԭ�����ӣ���һ��ΪFe2+���ӣ�����NO��������Լ����������ӵ�������ҺB�м������NaOH��Һ������GֻΪFe��OH��3����������F����FΪNH3��112mL���������ʵ�����0.005mol�����ݵ�Ԫ���غ㣬��Һ�к���NH4+�������ʵ�����0.005mol��Ũ����0.05mol/L����ҺH��ͨ��CO2���壬���ɳ���I����IΪAl��OH��3��HΪNaOH��NaAlO2��˵����Һ�к���Al3+���ӣ��ٸ������ӹ���֪ʶ����Һ�к���Fe2+���ӣ���һ������NO3-���ӣ�����SO42-���Ӿ�һ������Ba2+���ӣ�����ȷ���Ƿ��е�����Fe3+��Cl-��
=0.04mol��Ũ����0.04mol/0.1L=0.4mol/L����������A��A������������D��E����AΪNO��DΪNO2��EΪHNO3��˵����Һ�к��л�ԭ�����ӣ���һ��ΪFe2+���ӣ�����NO��������Լ����������ӵ�������ҺB�м������NaOH��Һ������GֻΪFe��OH��3����������F����FΪNH3��112mL���������ʵ�����0.005mol�����ݵ�Ԫ���غ㣬��Һ�к���NH4+�������ʵ�����0.005mol��Ũ����0.05mol/L����ҺH��ͨ��CO2���壬���ɳ���I����IΪAl��OH��3��HΪNaOH��NaAlO2��˵����Һ�к���Al3+���ӣ��ٸ������ӹ���֪ʶ����Һ�к���Fe2+���ӣ���һ������NO3-���ӣ�����SO42-���Ӿ�һ������Ba2+���ӣ�����ȷ���Ƿ��е�����Fe3+��Cl-��
��1���������Ϸ�����֪����Һ�в���ȷ����������ΪFe3+����Һ�в���ȷ����������ΪCl-�����������ӵķ���Ϊ��ȡ����B��Һ���Թ��У������еμ�AgNO3��Һ�����а�ɫ�������ɣ�˵������Cl-������Cl-���������ʴ�Ϊ��Fe3+��Cl-��ȡ����B��Һ���Թ��У������еμ�AgNO3��Һ�����а�ɫ�������ɣ�˵������Cl-������Cl-�����ڣ�
��2��FΪNH3������������������Ӧ�����Ȼ�狀͵�������Ӧ�Ļ�ѧ����ʽΪ��8NH3+3Cl2=6 NH4Cl+N2���ʴ�Ϊ��8NH3+3Cl2=6 NH4Cl+N2��
��3�����ݷ�Ӧ��3Fe2++NO3-+4H+=3Fe3++NO��+2H2O���õ�112mL0.005molNO��Fe2+�����ʵ�����0.015mol������ԭ��Һ��c��Fe2+��=0.015mol/0.1L=0.15mol/L����������������60mlʱ������������Ϊ0.025mol�����������������ʵ�����0.025mol��������Ԫ���غ㣬����Fe3+�����ʵ�����0.01mol������ԭ��Һ��c��Fe3+��=0.01mol/0.1L=0.1mol/L����������ӵ����ʵ���Ϊ![]() =0.04mol��Ũ����0.04mol/0.1L=0.4mol/L��NH4+�������ʵ�����0.005mol��Ũ����0.05mol/L��Al3+�����ʵ�����0.005mol��Ũ����0.05mol/L�����ݷ�Ӧ��3Fe2++NO3-+4H+=3Fe3++NO��+2H2O����֪�μӷ�ӦH+Ϊ0.005mol��4=0.02mol������������������H+Ϊ0.01L��2mol/L=0.02mol����ԭ��Һ��H+Ϊ0.02mol+0.02mol=0.04mol������2c��Fe2+��+3c��Fe3+��+3c��Al3+��+c��NH4+��=��2��0.15+3��0.1+3��0.05+0.05��mol/L=0.8mol/L��2c��SO42-��=0.8mol/L��ԭ��Һ�к���Cl-�����ݵ���غ㣺c��Cl-��=c��H+��=0.04mol/0.1L=0.4mol/L���ʴ�Ϊ��0.15�� 0.1��0.4��
=0.04mol��Ũ����0.04mol/0.1L=0.4mol/L��NH4+�������ʵ�����0.005mol��Ũ����0.05mol/L��Al3+�����ʵ�����0.005mol��Ũ����0.05mol/L�����ݷ�Ӧ��3Fe2++NO3-+4H+=3Fe3++NO��+2H2O����֪�μӷ�ӦH+Ϊ0.005mol��4=0.02mol������������������H+Ϊ0.01L��2mol/L=0.02mol����ԭ��Һ��H+Ϊ0.02mol+0.02mol=0.04mol������2c��Fe2+��+3c��Fe3+��+3c��Al3+��+c��NH4+��=��2��0.15+3��0.1+3��0.05+0.05��mol/L=0.8mol/L��2c��SO42-��=0.8mol/L��ԭ��Һ�к���Cl-�����ݵ���غ㣺c��Cl-��=c��H+��=0.04mol/0.1L=0.4mol/L���ʴ�Ϊ��0.15�� 0.1��0.4��

����Ŀ������̿�ɴ���������Ⱦ��NO��T��ʱ����1L�ܱ������м���NO�����̿�ۣ�������Ӧ������������A��B����ø����ʵ����ʵ������£�
����̿/mol | NO/mol | A/mol | B/mol | |
��ʼ״̬ | 2.030 | 0.100 | 0 | 0 |
2 minʱ | 2.000 | 0.040 | 0.030 | 0.030 |
��1��2 min�ڣ���NO��ʾ�÷�Ӧ��ƽ������v(NO)�� mol��L-1��min-1��
��2���÷�Ӧ�Ļ�ѧ����ʽ��________________________________________��
��3��һ�������£����ܱպ��ݵ������У��ܱ�ʾ������Ӧ�ﵽ��ѧƽ��״̬����_____ ��
��v(NO)��v(A)��v(B)=2��1��1
�����������ܶȲ��ٸı�
����ѹǿ���ٸı�
����������ƽ����Է����������ٸı�
��4��̼Ԫ�ؿ��γ������ڶ࣬�ֲ�������л���������м״��dz�����ȼ�ϣ��״�ȼ�ϵ�صĽṹʾ��ͼ���£�һ��ͨ��״�������һ��ͨ���������������Һ��ϡ���ᣬ��ع���ʱ�ܷ�Ӧʽ��2CH3OH��3O2��2CO2��4H2O��

��a��ͨ���������____���缫��ӦʽΪ��______��
��b���缫��ӦʽΪ_________________________��
�۵�ع���ʱH���� ������ ������������������