��Ŀ����
��һ����ĩ״���壬��������Al2��SO4��3��CuCl2��KNO3��Na2O�е�һ�ֻ�����ɵģ�Ϊ��̽�����ijɷ֣�ijѧϰС����������ʵ�飮
��ȡ������ĩͶ������ˮ�У���ĩ��ȫ�ܽ⣬�õ���ɫ����ҺA��
��ȡA��Һ��������������εμ��ռ���Һֱ���������۲쵽���г������֣�������������࣬Ȼ����������٣����������в�����ɫ������
����ȡA��Һ���ݣ�һ�ݵμ�BaCl2��Һ���ְ�ɫ��������һ�ݵμ���������Һ��Ҳ���ְ�ɫ������
��ش��������⣺
��1��ԭ��ɫ��ĩһ������______��һ������______�����ܺ���______�����ѧʽ��
��2��д�����̢����漰�������ӷ���ʽ��______��
��3�����Ҫ��һ��ȷ�����ܴ��ڵ����ʣ����е�ʵ�������ʵ������ͽ�����______��
��ȡ������ĩͶ������ˮ�У���ĩ��ȫ�ܽ⣬�õ���ɫ����ҺA��
��ȡA��Һ��������������εμ��ռ���Һֱ���������۲쵽���г������֣�������������࣬Ȼ����������٣����������в�����ɫ������
����ȡA��Һ���ݣ�һ�ݵμ�BaCl2��Һ���ְ�ɫ��������һ�ݵμ���������Һ��Ҳ���ְ�ɫ������
��ش��������⣺
��1��ԭ��ɫ��ĩһ������______��һ������______�����ܺ���______�����ѧʽ��
��2��д�����̢����漰�������ӷ���ʽ��______��
��3�����Ҫ��һ��ȷ�����ܴ��ڵ����ʣ����е�ʵ�������ʵ������ͽ�����______��
��ȡ������ĩͶ������ˮ�У���ĩ��ȫ�ܽ⣬�õ���ɫ����ҺA��˵������CuCl2����һ������Na2O���纬��Na2O���������������ͭ������
��ȡA��Һ��������������εμ��ռ���Һֱ���������۲쵽���г������֣�������������࣬Ȼ����������٣����������в�����ɫ������˵������Al2��SO4��3��
����ȡA��Һ���ݣ�һ�ݵμ�BaCl2��Һ���ְ�ɫ��������һ�ݵμ���������Һ��Ҳ���ְ�ɫ������˵����Һ�к�����������Ӻ������ӣ�����ȷ���Ƿ���KNO3��
��1�������Ϸ�����֪һ������CuCl2��Al2��SO4��3��һ��û��Na2O������ȷ������KNO3���ʴ�Ϊ��CuCl2��Al2��SO4��3��Na2O��KNO3��
��2����Һ�к���CuCl2�������������Ʒ���Cu2++2OH-=Cu��OH��2��������������ͭ����������Al2��SO4��3���������������ƣ�����Al3++3OH-=Al��OH��3��������Al��OH��3���������������������ƣ�����Al��OH��3�������ԣ������������Ʒ�Ӧ����ƫ�����ƺ�ˮ����Ӧ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ��Cu2++2OH-=Cu��OH��2����Al3++3OH-=Al��OH��3����Al��OH��3+OH-=AlO2-+2H2O��
��3����Ҫȷ���Ƿ�������أ��ɸ��ݼ����ӵ���ɫ��ӦΪ��ɫ���ýྻ�IJ���˿պȡԭ��Ʒ�ھƾ��ƻ��������գ�����ɫ�ܲ�Ƭ�۲������ɫ����Ϊ��ɫ����KNO3��������
�ʴ�Ϊ���ýྻ�IJ���˿պȡԭ��Ʒ�ھƾ��ƻ��������գ�����ɫ�ܲ�Ƭ�۲������ɫ����Ϊ��ɫ����KNO3��������
��ȡA��Һ��������������εμ��ռ���Һֱ���������۲쵽���г������֣�������������࣬Ȼ����������٣����������в�����ɫ������˵������Al2��SO4��3��
����ȡA��Һ���ݣ�һ�ݵμ�BaCl2��Һ���ְ�ɫ��������һ�ݵμ���������Һ��Ҳ���ְ�ɫ������˵����Һ�к�����������Ӻ������ӣ�����ȷ���Ƿ���KNO3��
��1�������Ϸ�����֪һ������CuCl2��Al2��SO4��3��һ��û��Na2O������ȷ������KNO3���ʴ�Ϊ��CuCl2��Al2��SO4��3��Na2O��KNO3��
��2����Һ�к���CuCl2�������������Ʒ���Cu2++2OH-=Cu��OH��2��������������ͭ����������Al2��SO4��3���������������ƣ�����Al3++3OH-=Al��OH��3��������Al��OH��3���������������������ƣ�����Al��OH��3�������ԣ������������Ʒ�Ӧ����ƫ�����ƺ�ˮ����Ӧ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ��Cu2++2OH-=Cu��OH��2����Al3++3OH-=Al��OH��3����Al��OH��3+OH-=AlO2-+2H2O��
��3����Ҫȷ���Ƿ�������أ��ɸ��ݼ����ӵ���ɫ��ӦΪ��ɫ���ýྻ�IJ���˿պȡԭ��Ʒ�ھƾ��ƻ��������գ�����ɫ�ܲ�Ƭ�۲������ɫ����Ϊ��ɫ����KNO3��������
�ʴ�Ϊ���ýྻ�IJ���˿պȡԭ��Ʒ�ھƾ��ƻ��������գ�����ɫ�ܲ�Ƭ�۲������ɫ����Ϊ��ɫ����KNO3��������

��ϰ��ϵ�д�
 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
�����Ŀ


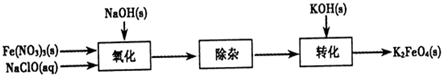
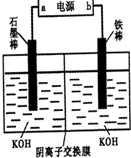
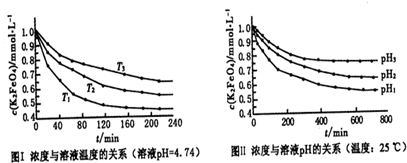
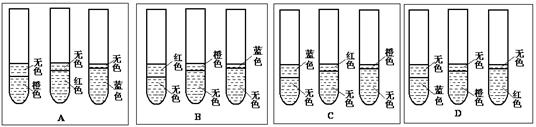
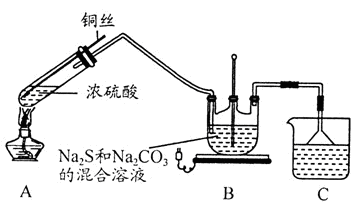
 3Na2S2O3+CO2���������װ��(�г�����ʡ��)����ʵ�顣
3Na2S2O3+CO2���������װ��(�г�����ʡ��)����ʵ�顣