��Ŀ����
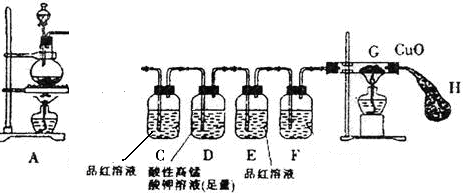
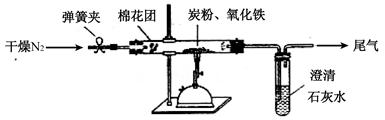
��12�֣�ijѧϰС��Ϊ֤�����۲�ͭ��ϡHNO3��Ӧ�IJ�����NO�����������ͼ��ʾ��ʵ��װ�á�����������ǵ�˼·���ش��йص����⡣

��һ��ʵ�����������Թܡ��������ܡ���Ƥ�����ձ�������ע������
������ʵ��ҩƷ��ͭ˿��ϡ���ᡢ̼��ƿ������ռ���Һ��
������ʵ��ԭ����
ͭ��ϡ���ᷴӦ�����ӷ�Ӧ����ʽ �� ��
���ģ�ʵ�鲽�裺
1������ͼ��ʾ���Ӻ�װ�ã�����װ�õ� �� ��
2�����Թ��м���һ�����Ĺ���ҩƷ���ѧʽ�� �� ��Ȼ�����Թ��е��������ϡ���ᣬ��Ѹ��������ͭ˿�͵��ܵ���Ƥ����
3�����Թ��еķ�Ӧ����һ��ʱ�����պ��NaOH��Һ�����ŷ�ס���ܿڣ�
4����ͭ˿�����ƶ������Թ�Һ���У�ʹ֮��ϡ���ᷴӦ��
5����ע��������ײ����Թܿڵ���Ƥ���У��������Թ������������
���壩ʵ�����ۣ�
1��ʵ�鲽��ڵ�Ŀ���ǣ�д����Ӧ�����ӷ���ʽ���������˵����
�� ��
2��ʵ�鲽��ݵ�Ŀ���ǣ�д����Ӧ�Ļ�ѧ����ʽ���������˵����
�� ��
������ʵ�����ۣ�
��װ�õ��ŵ��ǣ���дһ�����ɣ� �� ��
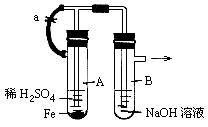
��ͬѧ�����������ͼ��ʾװ�ã��ڲ������ɺ������������ٳ�ȥ��װ�ã�����������۵ġ���պ��NaOH��Һ�����ŷ�ס���ܿڡ����������������� �� ��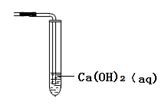

��һ��ʵ�����������Թܡ��������ܡ���Ƥ�����ձ�������ע������
������ʵ��ҩƷ��ͭ˿��ϡ���ᡢ̼��ƿ������ռ���Һ��
������ʵ��ԭ����
ͭ��ϡ���ᷴӦ�����ӷ�Ӧ����ʽ �� ��
���ģ�ʵ�鲽�裺
1������ͼ��ʾ���Ӻ�װ�ã�����װ�õ� �� ��
2�����Թ��м���һ�����Ĺ���ҩƷ���ѧʽ�� �� ��Ȼ�����Թ��е��������ϡ���ᣬ��Ѹ��������ͭ˿�͵��ܵ���Ƥ����
3�����Թ��еķ�Ӧ����һ��ʱ�����պ��NaOH��Һ�����ŷ�ס���ܿڣ�
4����ͭ˿�����ƶ������Թ�Һ���У�ʹ֮��ϡ���ᷴӦ��
5����ע��������ײ����Թܿڵ���Ƥ���У��������Թ������������
���壩ʵ�����ۣ�
1��ʵ�鲽��ڵ�Ŀ���ǣ�д����Ӧ�����ӷ���ʽ���������˵����
�� ��
2��ʵ�鲽��ݵ�Ŀ���ǣ�д����Ӧ�Ļ�ѧ����ʽ���������˵����
�� ��
������ʵ�����ۣ�
��װ�õ��ŵ��ǣ���дһ�����ɣ� �� ��
��ͬѧ�����������ͼ��ʾװ�ã��ڲ������ɺ������������ٳ�ȥ��װ�ã�����������۵ġ���պ��NaOH��Һ�����ŷ�ס���ܿڡ����������������� �� ��

�� 3Cu + 8H+ + 2NO3�� = 3Cu2+ + 2NO��+ 4H2O��2�֣� �������ԣ�1�֣� ��CaCO3��1�֣�
�� CaCO3 + 2H+ = Ca2+ + CO2��+ H2O ��2�֣�������CO2�ų��Թ��еĿ�������ֹO2��NO��Ӧ��1�֣�
�� 2NO + O2 = 2NO2��1�֣�,��������ɫ�����ɫ֤��Cu��ϡ���ᷴӦ����NO��1�֣�
��ʹ�ÿɳ鶯��ͭ˿����ʱ���Ʒ�Ӧ�Ŀ�ʼ�ͽ�������ԼҩƷ������������Ⱦ����IJ���������ʹ��պ��NaOH��Һ�����ŷ�ס���ܿڣ��ɷ�ֹNO��NO2�����ݳ���Ⱦ����������2�֣�
��ͨ��ʯ��ˮ����ǵ�����ȷ�ж��Թ��������Ž��в���ܣ�ʹ�ó��Ľ��۸���ѧ����1�֣�
�� CaCO3 + 2H+ = Ca2+ + CO2��+ H2O ��2�֣�������CO2�ų��Թ��еĿ�������ֹO2��NO��Ӧ��1�֣�
�� 2NO + O2 = 2NO2��1�֣�,��������ɫ�����ɫ֤��Cu��ϡ���ᷴӦ����NO��1�֣�
��ʹ�ÿɳ鶯��ͭ˿����ʱ���Ʒ�Ӧ�Ŀ�ʼ�ͽ�������ԼҩƷ������������Ⱦ����IJ���������ʹ��պ��NaOH��Һ�����ŷ�ס���ܿڣ��ɷ�ֹNO��NO2�����ݳ���Ⱦ����������2�֣�
��ͨ��ʯ��ˮ����ǵ�����ȷ�ж��Թ��������Ž��в���ܣ�ʹ�ó��Ľ��۸���ѧ����1�֣�
���������������ᣬ�ܺ�ͭ����������ԭ��Ӧ������ʽΪ3Cu + 8H+ + 2NO3�� = 3Cu2+ + 2NO��+ 4H2O��
������װ�����Ӻú����ȼ���װ�õ������ԡ�
������װ���к��п��������������ɵ�NO���Ӷ�����ʵ�飬������Ҫ���ž�װ���еĿ������������������̼��Ʒ�Ӧ���ɵ�CO2��ʵ�֣�������Ĺ���ҩƷ��̼��ƣ���Ӧ�ķ���ʽΪCaCO3 + 2H+ = Ca2+ + CO2��+ H2O��
������NO���ױ��������ɺ���ɫNO2������Ŀ����֤��Cu��ϡ���ᷴӦ����NO�ģ�����ʽΪ2NO + O2 = 2NO2��
������ʵ����ԴӲ��������׳̶ȡ�β���Ĵ�����ʵ������ȿ��ǡ�����װ�ü�ʵ����̿�֪���ŵ���ʹ�ÿɳ鶯��ͭ˿����ʱ���Ʒ�Ӧ�Ŀ�ʼ�ͽ�������ԼҩƷ������������Ⱦ����IJ���������ʹ��պ��NaOH��Һ�����ŷ�ס���ܿڣ��ɷ�ֹNO��NO2�����ݳ���Ⱦ��������
���������ſ���ʱ�����ж��Ƿ���ȫ�ž�������ͨ��ʯ��ˮ����ǵ�������ȷ�ж��Թ��������Ž��в���ܣ�ʹ�ó��Ľ��۸���ѧ��
������װ�����Ӻú����ȼ���װ�õ������ԡ�
������װ���к��п��������������ɵ�NO���Ӷ�����ʵ�飬������Ҫ���ž�װ���еĿ������������������̼��Ʒ�Ӧ���ɵ�CO2��ʵ�֣�������Ĺ���ҩƷ��̼��ƣ���Ӧ�ķ���ʽΪCaCO3 + 2H+ = Ca2+ + CO2��+ H2O��
������NO���ױ��������ɺ���ɫNO2������Ŀ����֤��Cu��ϡ���ᷴӦ����NO�ģ�����ʽΪ2NO + O2 = 2NO2��
������ʵ����ԴӲ��������׳̶ȡ�β���Ĵ�����ʵ������ȿ��ǡ�����װ�ü�ʵ����̿�֪���ŵ���ʹ�ÿɳ鶯��ͭ˿����ʱ���Ʒ�Ӧ�Ŀ�ʼ�ͽ�������ԼҩƷ������������Ⱦ����IJ���������ʹ��պ��NaOH��Һ�����ŷ�ס���ܿڣ��ɷ�ֹNO��NO2�����ݳ���Ⱦ��������
���������ſ���ʱ�����ж��Ƿ���ȫ�ž�������ͨ��ʯ��ˮ����ǵ�������ȷ�ж��Թ��������Ž��в���ܣ�ʹ�ó��Ľ��۸���ѧ��

��ϰ��ϵ�д�
 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�
�����Ŀ

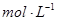 NaOH��Һ�У��ж�п����ȫ��Ӧ�ı�־�ǣ�__________�����ȡ����Ƭ��ˮ��ϴ����ɺ������������Ϊm2����п�Ʋ㵥����Ϊh��п���ܶ�Ϊ
NaOH��Һ�У��ж�п����ȫ��Ӧ�ı�־�ǣ�__________�����ȡ����Ƭ��ˮ��ϴ����ɺ������������Ϊm2����п�Ʋ㵥����Ϊh��п���ܶ�Ϊ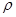 ����h=__________
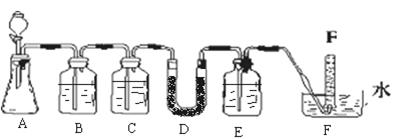
����h=__________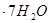 �����IJ����ǣ���1g�̷�����30mL����ˮ�ܽⲢ����H2SO4��Һ��H3PO4��Һ������0.02mol/L KMnO4����Һ�ζ�����Һ�ձ�ɷۺ�ɫ��ֹͣ�ζ������ı���ҺVmL����Ӧ���漰����Ҫ��ѧ����ʽ�У�
�����IJ����ǣ���1g�̷�����30mL����ˮ�ܽⲢ����H2SO4��Һ��H3PO4��Һ������0.02mol/L KMnO4����Һ�ζ�����Һ�ձ�ɷۺ�ɫ��ֹͣ�ζ������ı���ҺVmL����Ӧ���漰����Ҫ��ѧ����ʽ�У�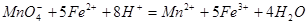
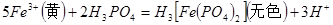
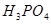 ��������__________��
��������__________��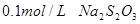 ��Һ��
��Һ��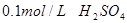 ���ձ�����ˮ����ˮ�������
���ձ�����ˮ����ˮ�������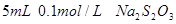 ��
��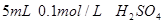 ��
��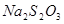 ��
��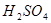 ��Ӧ�Ļ�ѧ����ʽΪ ��
��Ӧ�Ļ�ѧ����ʽΪ ��