��Ŀ����
������������Ǻ���һ�����һ�������ˮƽ����Ҫ��־��
��1��һ�������£�SO2�������Ӧ10 min��SO2��SO3���ʵ���Ũ�ȷֱ�Ϊ1.2 mol/L��2.0 mol/L����SO2��ʼ���ʵ���Ũ��Ϊ______������SO3�Ļ�ѧ��Ӧ����Ϊ______��
��2�����ı��������������SO2�������Ӧ����SO3��ʹ10 min�ڵ���O2��ʾ�ķ�Ӧ����Ϊ0.15mol/(L��min)����ı������������_______________��
| A��ѹ�����������ѹǿ | B�������¶� | C����������ĵ��� | D��������SO2��Ũ�� |
(1) 3.2 mol/L 0.2 mol/(L��min) ��(2) A D �� (3) SO2 + 2NH3��H2O = 2NH4��+ SO32��+ H2O
���������������1�����ݷ�Ӧ����ʽ2SO2(g) +O2(g) 2SO3(g)��֪������SO3���ʵ���Ũ����2.0 mol/L�������ĵ�SO2�����ʵ���Ũ����2.0 mol/L����ʱ������SO2 1.2 mol/L�����Կ�ʼʱSO2�����ʵ���Ũ���ǣ�2.0 +1.2��mol/L="3.2mol/L." ����SO3�Ļ�ѧ��Ӧ����ΪV(SO3)=��c�¦�t="2.0" mol/L��10 min=" 0.2" mol/(L��min)����2��V(O2): V(SO3)=1:2������V(O2)= 0.1mol/(L��min).�������ı�����ʹV(O2)=" 0.15" mol/(L��min)��A��ѹ�����������ѹǿ����ʹ���ʵ�Ũ�������ܴﵽV(O2)= 0.1mol/(L��min)����ȷ��B���������¶ȣ���Ӧ���ʼ���������C����������ĵ����������������ݻ����䣬������ʵķ�Ӧ���ʲ��䡣����D��������SO2��Ũ�ȣ�����Ӧ���Ũ�Ȼ�ѧ��Ӧ���ʼӿ죬��ȷ����ѡ��ΪA��D����3��SO2������������ܹ�������Ӧ�����κ�ˮ������ڹ�ҵ�����ᣬ�����ù����İ�ˮ��SO2β�����������õ����Σ������Ӧ�����ӷ���ʽ��SO2 + 2NH3��H2O = 2NH4��+ SO32��+ H2O��
2SO3(g)��֪������SO3���ʵ���Ũ����2.0 mol/L�������ĵ�SO2�����ʵ���Ũ����2.0 mol/L����ʱ������SO2 1.2 mol/L�����Կ�ʼʱSO2�����ʵ���Ũ���ǣ�2.0 +1.2��mol/L="3.2mol/L." ����SO3�Ļ�ѧ��Ӧ����ΪV(SO3)=��c�¦�t="2.0" mol/L��10 min=" 0.2" mol/(L��min)����2��V(O2): V(SO3)=1:2������V(O2)= 0.1mol/(L��min).�������ı�����ʹV(O2)=" 0.15" mol/(L��min)��A��ѹ�����������ѹǿ����ʹ���ʵ�Ũ�������ܴﵽV(O2)= 0.1mol/(L��min)����ȷ��B���������¶ȣ���Ӧ���ʼ���������C����������ĵ����������������ݻ����䣬������ʵķ�Ӧ���ʲ��䡣����D��������SO2��Ũ�ȣ�����Ӧ���Ũ�Ȼ�ѧ��Ӧ���ʼӿ죬��ȷ����ѡ��ΪA��D����3��SO2������������ܹ�������Ӧ�����κ�ˮ������ڹ�ҵ�����ᣬ�����ù����İ�ˮ��SO2β�����������õ����Σ������Ӧ�����ӷ���ʽ��SO2 + 2NH3��H2O = 2NH4��+ SO32��+ H2O��
���㣺���黯ѧ��Ӧ�в�ͬ���ʼ��Ũ�ȹ�ϵ����ѧ��Ӧ���ʵļ����Ӱ�����ء����ӷ���ʽ����д��

��5�֣�������ũҵ������ҵ���к���Ҫ�����ã���ʷ��ŵ������ѧ��������3�ΰ���о������������ϳɰ��Ļ�ѧ�ҡ�
��1����ͼ��ʾ�¶�ΪT Kʱ�����������ϳɰ���Ӧ�����е������仯�� д���÷�Ӧ���Ȼ�ѧ����ʽ ��
��2����֪��TK�¶��ºϳɰ���Ӧ��2.00L���ܱ������н��С��õ��������ݣ�
| ʱ�䣨h�� ���ʵ�����mol�� | | 0 | 1 | 2 | 3 | 4 |
| N2 | | 1.50 | n1 | 1.20 | n3 | 1.00 |
| H2 | | 4.50 | 4.20 | 3.60 | n4 | 3.00 |
| NH3 | | 0.00 | 0.20 | n2 | 1.00 | 1.00 |
���ݱ������ݼ��㣺
��0~1h��N2��ƽ����Ӧ����Ϊmol/(L��h)��
�ڷ�Ӧ���е�2hʱ�ų�������Ϊ kJ��
�۴������£���Ӧ��N2 + 3H2
 2NH3�Ļ�ѧƽ�ⳣ��K = ��������λС������
2NH3�Ļ�ѧƽ�ⳣ��K = ��������λС�������ܷ�Ӧ�ﵽƽ�������ƽ����ϵ���ټ���N2��H2��NH3��1mol����ѧƽ���ƶ��ķ����� �������Ӧ�����淴Ӧ�����ƶ�������
(14��)��ѧ��һֱ�������о����¡���ѹ�¡��˹��̵������·���������ʵ�鱨�����ڳ��¡���ѹ�����������£�N2�ڴ���(��������Fe2O3��TiO2)������ˮ������Ӧ�����ɵ���Ҫ����ΪNH3����һ���о�NH3���������¶ȵĹ�ϵ������ʵ�����ݼ��±�(���ա�N2ѹǿ1.0��105 Pa����Ӧʱ��3 h)��
| T��K | 303 | 313 | 323 | 353 |
| NH3������/(10-6mol) | 4.8 | 5.9 | 6.0 | 2.0 |
��ش��������⣺
(1)���ڷ����ڵ�����ϵ�У�������Ӧ(I)���д�����������������·�Ӧ��������ϵ�����仯ʾ��ͼ�������б�Ҫ��ע��
(2)��Ŀǰ�㷺ʹ�õĹ�ҵ�ϳɰ�������ȣ��÷����й̵���Ӧ�������������������䷴Ӧ����������NH3���������Ľ��飺 ��
(3)д����ҵ����H2��N2ֱ�Ӻϳ�NH3�Ļ�ѧ����ʽ ������2.0 L���ܱ������г���0.60mol N2(g)��1.60mol H2(g)����Ӧ��һ�������´ﵽƽ��ʱ��NH3�����ʵ�������(NH3�����ʵ����뷴Ӧ��ϵ�������ʵ���֮��)Ϊ4/7�������������N2��ƽ��ת����Ϊ ����Ӧ��ƽ�ⳣ��K�� (��Ҫ��д��λ)��
����16�֣�����֪���з�Ӧ���Ȼ�ѧ����ʽΪ��
��1�� C(s) + O2(g) = CO2(g) ��H1 =" -393.5" kJ/mol
��2�� CH3COOH(l) + 2O2(g) = 2CO2(g) + 2H2O(l) ��H2 =" -870.3" kJ/mol
��3�� 2H2(g) + O2(g) = 2H2O(l) ��H3 =" -571.6" kJ/mol
�����:2C(s) + 2H2(g) + O2(g)= CH3COOH(l) ��H4 = ��
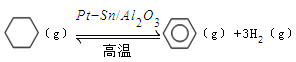
����ij�¶��£�����(t��BuNO)2���������CCl4�ܼ��о����Է�����Ӧ��
(t��BuNO)2 ��2(t��BuNO) �����¶��¸÷�Ӧ��CCl4�ܼ��е�ƽ�ⳣ��Ϊ1.4��
��2(t��BuNO) �����¶��¸÷�Ӧ��CCl4�ܼ��е�ƽ�ⳣ��Ϊ1.4��
��1����1L�������м���0.50mol��t-BuNO��2��10minʱ��Ӧ��ƽ�⣬��ʱ��t-BuNO��2��ƽ��ת����Ϊ60%�����跴Ӧ��������Һ���ʼ��Ϊ1L������Ӧ��ǰ10min�ڵ�ƽ������Ϊ�ͣ�t-BuNO��= ����ʽ����������Ӧ��ƽ�ⳣ��K =������ �� ��
��2���йط�Ӧ��(t��BuNO)2 2(t��BuNO) ��������ȷ���ǣ� ��
2(t��BuNO) ��������ȷ���ǣ� ��
A��ѹǿԽ��Ӧ���ת����Խ�� B���¶����ߣ���ƽ��һ�������ƶ�
C���ܼ���ͬ��ƽ�ⳣ��Kֵ��ͬ
��3��ͨ����ɫ�����õ�40��ʱ(t��BuNO)2��(t��BuNO)Ũ����ʱ��ı仯��ϵ�ļ����������±���ʾ������ͬһͼ�л��(t��BuNO)2�ͣ�t��BuNO��Ũ����ʱ��ı仯���ߡ�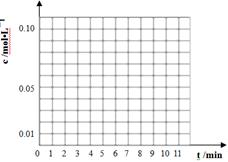
| ʱ�䣨min�� | 0 | 1 | 3 | 5 | 7 | 9 | 11 |
| c(t��BuNO)2 mol/L | 0.05 | 0.03 | 0.01 | 0.005 | 0.003 | 0.002 | 0.002 |
| c(t��BuNO) mol/L | 0 | 0.04 | 0.08 | 0.07 | 0.094 | 0.096 | 0.096 |

 ��ij�¶��£�������ܱ������м��뻷���飬��ʼŨ��Ϊa mol/L��ƽ��ʱ����Ũ��Ϊbmol/L���÷�Ӧ��ƽ�ⳣ��K��_____��
��ij�¶��£�������ܱ������м��뻷���飬��ʼŨ��Ϊa mol/L��ƽ��ʱ����Ũ��Ϊbmol/L���÷�Ӧ��ƽ�ⳣ��K��_____��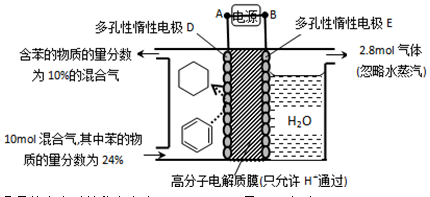
 ��_____��
��_____�� ��100%������������С�����1λ��
��100%������������С�����1λ��



 H=-1266.8kJ��mol-1
H=-1266.8kJ��mol-1
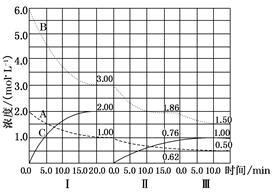
 cC(g)(��H<0)�ڵ��������½��С��ı�������Ӧ�������ڢ����ϵ�и�����Ũ����ʱ��仯��������ͼ��ʾ��
cC(g)(��H<0)�ڵ��������½��С��ı�������Ӧ�������ڢ����ϵ�и�����Ũ����ʱ��仯��������ͼ��ʾ��