��Ŀ����
��14�֣���������Ҫ������Դ��������Ϊ�������õĹؼ��������ǵ�ǰ��ע���ȵ�֮һ��
��1�������������Դ����ȼ�ղ���Ϊ__________��
��2��NaBH4��һ����Ҫ�Ĵ������壬����ˮ��Ӧ�ﵽNaBO2���ҷ�Ӧǰ��B�Ļ��ϼ۲��䣬�÷�Ӧ�Ļ�ѧ����ʽΪ___________����Ӧ����1mol NaBH4ʱת�Ƶĵ�����ĿΪ__________��
��3������ɽ����л�������û�����ͱ�֮��Ŀ��淴Ӧ��ʵ������ͼ��⣺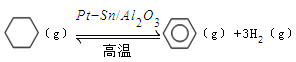 ��ij�¶��£�������ܱ������м��뻷���飬��ʼŨ��Ϊa mol/L��ƽ��ʱ����Ũ��Ϊbmol/L���÷�Ӧ��ƽ�ⳣ��K��_____��
��ij�¶��£�������ܱ������м��뻷���飬��ʼŨ��Ϊa mol/L��ƽ��ʱ����Ũ��Ϊbmol/L���÷�Ӧ��ƽ�ⳣ��K��_____��
��4��һ�������£���11ͼʾװ�ÿ�ʵ���л���ĵ绯ѧ���⣨���������л����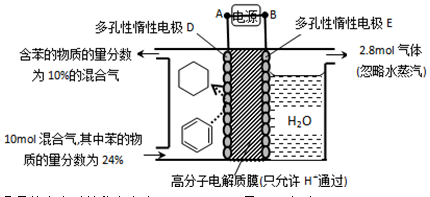
�ٵ����е����ƶ�����Ϊ____________��
������Ŀ�����ĵ缫��ӦʽΪ_________��
�۸ô���װ�õĵ���Ч��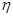 ��_____��
��_____��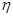 ��
�� ��100%������������С�����1λ��
��100%������������С�����1λ��
��1��ˮ��H2O ��2��NaBH4��2H2O=NaBO2��4H2����4NA��2.408��1024
��3��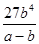 mol3/L3 ��4����A��D ��C6H6��6H����6e����C6H12 ��64.3%
mol3/L3 ��4����A��D ��C6H6��6H����6e����C6H12 ��64.3%
���������������1��������ȼ�ղ�����ˮ��
��2����Ӧǰ��BԪ�صĻ��ϼ۲��䣬��Ӧǰ��BԪ�صĻ��ϼ۾��ǣ�3�ۣ���˷�ӦǰNaBH4����Ԫ�صĻ��ϼ��ǣ�1�ۡ�ˮ����Ԫ�صĻ��ϼ��ǣ�1�ۣ���˷�Ӧ�л����������ɣ���Ӧ�Ļ�ѧ����ʽΪNaBH4��2H2O=NaBO2��4H2����NaBH4����Ԫ�صĻ��ϼ۴ӣ�1�����ߵ�0�ۣ����1molNaBH4�ڷ�Ӧ��ʧȥ4mol���ӣ�����Ŀ��4NA��2.408��1024��
��3��ƽ��ʱ����Ũ����b mol/L������ݷ�Ӧ�ķ���ʽ��֪���Ļ������Ũ����b mol/L������������Ũ����3 b mol/L����ƽ��ʱ�������Ũ��Ϊ��a��b��mol/L�����ڻ�ѧƽ�ⳣ������һ�������£������淴Ӧ�ﵽƽ��״̬ʱ��������Ũ�ȵ���֮���ͷ�Ӧ��Ũ�ȵ���֮���ı�ֵ������¶��·�Ӧ��ƽ�ⳣ��Ϊ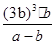 ��
��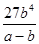 mol3/L3��
mol3/L3��
��4���ٱ����ɻ��������ڵ��ⷴӦ������ǻ�ԭ��Ӧ�����缫D���������缫E����������˵����е��ӵ�����������A��D��
�ڱ��õ��������ɻ�������Ŀ�������ڴ������ӽ���Ĥ�������������������ƶ�����缫��ӦʽΪC6H6��6H����6e����C6H12��
����������2.8mol���壬������Ӧ��������OH���ŵ����ɵ���������ת�Ƶ��ӵ����ʵ�����2.8mol��4��11.2mol�����������ı������ʵ�����xmol����ͬʱ���� xmol�����飬���ݵ缫��ӦʽC6H6��6H����6e����C6H12��֪�õ�������6xmol�����ݵ����غ��֪����������������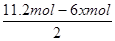 ��5.6mol��3xmol������
��5.6mol��3xmol������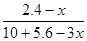 ��0.1�����x��1.2����˴���װ�õĵ���Ч�ʣ�
��0.1�����x��1.2����˴���װ�õĵ���Ч�ʣ� ��100%��64.3%��
��100%��64.3%��
���㣺����������ԭ��Ӧ����ʽ��ƽ�ͼ��㡢ƽ�ⳣ�������Լ��绯ѧԭ����Ӧ�������

��8�֣���1������δ����õ���Դѡ����ȡ�����ij���ķ����кܶ࣬���ü״�������ˮ������Ӧ������������Ӧ����ʽ���£�
CH3OH(g) + H2O(g)  CO2(g) + 3H2(g)
CO2(g) + 3H2(g) 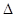 H(298K)��+ 49.4 kJ/mol
H(298K)��+ 49.4 kJ/mol
һ�������£����ݻ�Ϊ2L�ĺ����ܱ������г���1 mol CH3OH(g)��3 mol H2O(g)��ʵ���ã��ﵽƽ��״̬ʱ����������19.76 kJ����
�ٴ�ƽ��ʱ��������ѹǿ�Ƿ�Ӧǰ�� ����
�ڸ������µĸ÷�Ӧ��ƽ�ⳣ���� �����������λ��Ч���֣���
�۸������·�Ӧ��ƽ��״̬�������ǣ�����ţ� ��
| A��v��(CH3OH)��v��(CO2) | B�����������ܶȲ��� |
| C��c(CH3OH)��c(H2O) | D���������������ʵ������� |
 2SO3(g)
2SO3(g) 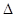 H��0���ס�����ʼ��Ӧ�¶���ͬ���������������������ƽ��ʱ������SO2��ת����Ϊa������SO3�ķֽ���Ϊb����a��b�Ĺ�ϵΪa+b _______ 1�������������������) ��
H��0���ס�����ʼ��Ӧ�¶���ͬ���������������������ƽ��ʱ������SO2��ת����Ϊa������SO3�ķֽ���Ϊb����a��b�Ĺ�ϵΪa+b _______ 1�������������������) �� ��12�֣���ѧ��һֱ�����ڡ��˹��̵����ķ����о���
��1���ϳɰ���ԭ��Ϊ��N2(g)+3H2(g)  2NH3(g)
2NH3(g)  H="-92.4" kJ��mol���÷�Ӧ�������仯��ͼ��ʾ��
H="-92.4" kJ��mol���÷�Ӧ�������仯��ͼ��ʾ��
���ڷ�Ӧ��ϵ�м����������Ӧ��������E2�ı仯�� (���������С�����䡱)��
�ڽ�0.3 mol N2��0.5 mol H2�������������ܱ������У���һ�������´ﵽƽ�⣬�������������ѹǿ��Ϊԭ���� ����ʱH2��ת����Ϊ ������������������߸�������H2��ת���ʣ����д�ʩ���е��� (��ѡ����ĸ)��
����ʱH2��ת����Ϊ ������������������߸�������H2��ת���ʣ����д�ʩ���е��� (��ѡ����ĸ)��
| A���������а�ԭ�����ٳ���ԭ���� | B�����������ٳ���һ����H2 |
| C���ı䷴Ӧ�Ĵ��� | D��Һ�������������� |
2N2(g)+6H2O��1��
 4NH3(g)+3O2(g)
4NH3(g)+3O2(g)  H="+1530" kJ��mol
H="+1530" kJ��mol��֪��H2O��1��=H2O(g)
 H="+44.0" kJ��mol
H="+44.0" kJ��mol��2N2(g)+6H2O(g)
 4NH3(g)+3O2(g)
4NH3(g)+3O2(g)  H = kJ��mol���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK= �����������������䣬����ѹǿ��Kֵ (���������С�����䡱)��
H = kJ��mol���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK= �����������������䣬����ѹǿ��Kֵ (���������С�����䡱)�� ������������Ǻ���һ�����һ�������ˮƽ����Ҫ��־��
��1��һ�������£�SO2�������Ӧ10 min��SO2��SO3���ʵ���Ũ�ȷֱ�Ϊ1.2 mol/L��2.0 mol/L����SO2��ʼ���ʵ���Ũ��Ϊ______������SO3�Ļ�ѧ��Ӧ����Ϊ______��
��2�����ı��������������SO2�������Ӧ����SO3��ʹ10 min�ڵ���O2��ʾ�ķ�Ӧ����Ϊ0.15mol/(L��min)����ı������������_______________��
| A��ѹ�����������ѹǿ | B�������¶� | C����������ĵ��� | D��������SO2��Ũ�� |

 CH3OCH3(g)��3H2O(g)����H
CH3OCH3(g)��3H2O(g)����H


 Fe(s)��CO2(g)��ƽ�ⳣ��K��0.25��
Fe(s)��CO2(g)��ƽ�ⳣ��K��0.25��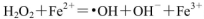
 ����Fe2���ڴ˹����������������______________��������336mL O2����״����ʱ����Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ_______mol��
����Fe2���ڴ˹����������������______________��������336mL O2����״����ʱ����Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ_______mol��


 .
. 2NH3��2 min����������������1mol NH3������
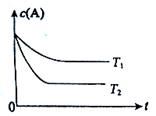
2NH3��2 min����������������1mol NH3������ B(g) +C(g)���ݻ�Ϊ1.0L���ܱ������н��У�A�ij�ʼŨ��Ϊ0.050mol/L���¶�T1��T2��A��Ũ����ʱ���ϵ��ͼ��ʾ���ش��������⣺
B(g) +C(g)���ݻ�Ϊ1.0L���ܱ������н��У�A�ij�ʼŨ��Ϊ0.050mol/L���¶�T1��T2��A��Ũ����ʱ���ϵ��ͼ��ʾ���ش��������⣺