��Ŀ����
6�� �о���Աͨ���Ա�������PM2.5�Ļ�ѧ����о����֣�����β����ȼú��Ⱦ�ֱ�ռ4%��18%��
�о���Աͨ���Ա�������PM2.5�Ļ�ѧ����о����֣�����β����ȼú��Ⱦ�ֱ�ռ4%��18%����1����ϡ���ȴ����ܽ�����β���е�CO��NOx̼�⻯����ת���������ʣ��Ӷ���������β����Ⱦ����֪��
N2��g��+O2��g��=2NO��g����H=+180.5kJ•mol-1
2C��s��+O2��g��=2CO��g����H=-221.0kJ•mol-1
C��s��+O2��g��=CO2��g����H=-393.5kJ•mol-1
д��NO��g����CO��g����ת����N2��g����CO2��g�����Ȼ�ѧ����ʽ��2NO ��g��+2CO��g��=N2��g��+2CO2��g����H=-746.5 kJ/mol��
��2���������û���̿��ԭ���������������ӦΪC��s��+2NO��g��?N2��g��+CO2��g��H����ij�ܱ���������һ�����Ļ���̿��NO�����£�T1�棩�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
| ������Ũ��/mol•L-1 ʱ��/min | NO | N2 | CO2 |
| 0 | 0.100 | 0 | 0 |
| 10 | 0.058 | 0.021 | 0.021 |
| 20 | 0.050 | 0.025 | 0.025 |
| 30 | 0.050 | 0.025 | 0.025 |
| 40 | 0.036 | 0.032 | 0.010 |
| 50 | 0.036 | 0.032 | 0.010 |
��ǰ10min����v��NO����ʾ�Ļ�ѧ��Ӧ����Ϊ0.0042mol/��L��min����30min�ı�ijһ��������Ӧ���´ﵽƽ�⣬��ı�����������ǽ���CO2Ũ�ȣ�
����30min�������¶���T2�棬�ﵽƽ��ʱ��������NO��N2��CO2Ũ��֮��Ϊ3��1��1����÷�Ӧ�ġ�H��0���������=����������
��3���������崫�������Լ������β����CO�ĺ���������������ȼ�ϵ��Ϊ����ԭ������װ����ͼ��ʾ���õ���е����Ϊ������-�����ƣ�����O2-�����ڹ������NASICON�������ƶ���aΪ�������缫��ӦʽΪCO-2e-+O2-�TCO2����ع��������У�O2-��b�����a����b������ͬ������a����
��ŷIV��������֤������һ�����ŷ���ֵ�����ͻ�CO1.00g/km��ij���Գ��������У�ÿ��ʻ1km��������ͨ������Ϊ0.08mol����ó������ϣ�����ϡ������ϡ���ŷIV�ŷű���
���� ��1�����ݷ���ʽ�ļӼ��ó�NO��CO��ת����N2��CO2�Ļ�ѧ����ʽ���ʱ���Ӧ�ļӼ����Ӷ��ó����Ȼ�ѧ��Ӧ����ʽ��
��2����20minʱ��Ӧ�õ�ƽ�⣬��ƽ��Ũ�ȴ���ƽ�ⳣ��K=$\frac{c��{N}_{2}����c��C{O}_{2}��}{{c}^{2}��NO��}$���㣻
�ڸ���v=$\frac{��c}{��t}$����v��NO����
30min�ı���������40min�ֵ���ƽ�⣬NO��CO2Ũ�ȼ�С��N2��Ũ��Ũ������ƽ��������Ӧ�����ƶ����ұ���NO��N2Ũ�ȱ仯��֮��Ϊ��0.05-0.036������0.032-0.025��=2��1�����ڻ�ѧ������֮�ȣ���Ӧ�ǽ���CO2Ũ�ȣ�
��30min����ƽ��״̬��NO��N2��CO2Ũ��֮��Ϊ2��1��1����30min�������¶���T2�棬�ﵽƽ��ʱ��������NO��N2��CO2Ũ��֮��Ϊ3��1��1��˵��ƽ�����淴Ӧ�����ƶ���
��3���ٱ�����CO��������Ӧ����CO2��CO��a������������Ӧ����aΪ������bΪ���������������ƶ���O2-�ɵ缫b����缫a��
�ڸ���ת�Ƶ��Ӽ�����ʻ1Kmʱ�ŷ�CO�������������ж��Ƿ�����ŷű���
��� �⣺��1����֪���٣�N2��g��+O2��g��=2NO��g����H=+180.5kJ/mol��
�ڣ�2C��s��+O2��g��=2CO��g����H=-221.0kJ/mol��
�ۣ�C��s��+O2��g��=CO2��g����H=-393.5kJ/mol ��
���ݸ�˹���ɣ��ۡ�2-��-�ٿɵã�NO ��g��+2CO��g��=N2��g��+2CO2��g�������ԡ�H=��-393.5kJ/mol����2-��-221.0kJ/mol��-��+180.5kJ/mol��=-746.5 kJ/mol��
�ʴ�Ϊ��2NO ��g��+2CO��g��=N2��g��+2CO2��g����H=-746.5 kJ/mol��
��2����20minʱ��Ӧ�õ�ƽ�⣬��ƽ��Ũ�ȴ�����㣬��֪K=$\frac{c��{N}_{2}����c��C{O}_{2}��}{{c}^{2}��NO��}$=$\frac{0.025��0.025}{0.0{5}^{2}}$=0.25��
�ʴ�Ϊ��0.25��
�ڸ���v=$\frac{��c}{��t}$��֪��v��NO��=$\frac{0.1mol/L-0.058mol/L}{10min}$=0.0042mol/��L��min����
30min�ı���������40min�ֵ���ƽ�⣬NO��CO2Ũ�ȼ�С��N2��Ũ��Ũ������ƽ��������Ӧ�����ƶ����ұ���NO��N2Ũ�ȱ仯��֮��Ϊ��0.05-0.036������0.032-0.025��=2��1�����ڻ�ѧ������֮�ȣ���Ӧ�ǽ���CO2Ũ�ȣ�
�ʴ�Ϊ��0.0042mol/��L��min��������CO2Ũ�ȣ�
��30min����ƽ��״̬��NO��N2��CO2Ũ��֮��Ϊ2��1��1����30min�������¶���T2�棬�ﵽƽ��ʱ��������NO��N2��CO2Ũ��֮��Ϊ3��1��1��˵��ƽ�����淴Ӧ�����ƶ�����÷�Ӧ�ġ�H��0��
�ʴ�Ϊ������
��3���ٱ�����CO��������Ӧ����CO2��CO��a������������Ӧ����aΪ������bΪ���������������ƶ���O2-�ɵ缫b����缫a�������缫��ӦʽΪ��CO-2e-+O2-�TCO2��
�ʴ�Ϊ������CO-2e-+O2-�TCO2��b��a��
��ÿ��ʻ1km��������ͨ������Ϊ0.08mol����CO-2e-+O2-�TCO2��֪���ŷ�CO������Ϊ0.08mol��$\frac{1}{2}$��28g/mol=1.12g��1.00g���������ŷű���
�ʴ�Ϊ�������ϣ�
���� ���⿼���Ȼ�ѧ����ʽ��д��ƽ�ⳣ�����㡢��Ӧ���ʼ��㡢��ѧƽ���ƶ���ԭ��ؼ������ȣ�����ƴ������Ŀ���Ƕ�ѧ���ۺ������Ŀ��飬�Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�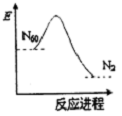 �����ѧ��ȷ�ϣ�������һ�־��п��ģ�������������ṹ�ķ���N60��N60ת��N2�������仯��ͼ��ʾ������˵����ȷ���ǣ�������
�����ѧ��ȷ�ϣ�������һ�־��п��ģ�������������ṹ�ķ���N60��N60ת��N2�������仯��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | N60ת��N2�������仯 | B�� | N2����N60�ȶ� | ||
| C�� | N60ת��N2�����������Ĺ��� | D�� | N60���ܳ�Ϊһ�ֺõĻ������ |
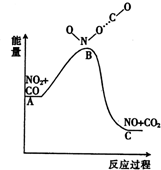
| A�� | �÷�ӦΪ���ȷ�Ӧ | |
| B�� | �÷�ӦΪ��������ԭ��Ӧ | |
| C�� | NO��CO2�ļ����ܺʹ���NO2��CO�ļ����ܺ� | |
| D�� | 1 molNO2��g����1 mol CO��g�������������� 1mol NO��g����1 molCO2��g���������� |

��֪��NaClO2���ܽ�����¶����߶������ʵ������¿ɽᾧ����NaClO2•3H2O��
��1���������ڷ�����Ӧ�Ļ�ѧ����ʽΪ2NaOH+2ClO2+H2O2=2NaClO2+2H2O+O2���ù��������е�NaClO3��ClO2��NaClO2����ǿ�����������Ƕ��ܺ�Ũ���ᷴӦ��ȡCl2�����ö������Ⱥ�Ũ������ȡCl2��������5mol Cl2ʱ��ͨ����ԭ��Ӧ�Ƶ�����������Ϊ71�ˣ�
��2������Һ�еõ���NaClO2•3H2O����IJ���������dc����д��ţ���
a������ b������ c������ d����ȴ�ᾧ e����������fϴ�ӡ�g ����
��3��ӡȾ��ҵ�����������ƣ�NaClO2��Ư��֯�Ư��֯��ʱ���������õ���HClO2���±��� 25��ʱHClO2�����ֳ�������ĵ���ƽ�ⳣ����
| ���� | HClO2 | HF | HCN | HS |
| Ka/mol•L-1 | 1��10-2 | 6.3��10-4 | 4.9��10-10 | K1=9.1��10-8 K2=1.1��10-12 |
��Na2S�dz��õij�������ij��ҵ��ˮ�к��е�Ũ�ȵ�Cu2+��Fe2+��Pb2+���ӣ��μ�Na2S��Һ�����������ij�����CuS�������һ�����ӳ�����ȫʱ��������Ũ��Ϊ10-5mol•L-1����ʱ��S2-��Ũ��Ϊ6.3��10-13mol/L��
��Ϊ����ҵ��ˮ�е�Cu2+��Pb2+��Hg2+�������ӣ���������˹�ҵ��ˮ�м���������Լ���B
A��NaOH B FeS C Na2S
��֪Ksp��FeS��=6.3��10-10��Ksp��CuS��=6��10-36��Ksp��PbS��=2.4��10-28��
 ������ȫ�����س��ڱ��������֣���������������β��ռ20%���ϣ���֪����β���е���Ҫ��Ⱦ��ΪNOx��CO��ȼ��Դ��ϸ������PM2.5�����ɺ����ʣ�Ŀǰ�����о����˶�����������β����Ⱦ�ķ�����
������ȫ�����س��ڱ��������֣���������������β��ռ20%���ϣ���֪����β���е���Ҫ��Ⱦ��ΪNOx��CO��ȼ��Դ��ϸ������PM2.5�����ɺ����ʣ�Ŀǰ�����о����˶�����������β����Ⱦ�ķ�������1����������ʱ��H2��NO��ԭΪN2��
��֪��

��H2��ԭNO���ɵ�����ˮ�������Ȼ�ѧ����ʽ�ǣ�2NO��g��+2H2��g���TN2��g��+2H2O��g����H=-665 kJ•mol-1��
��2���û���̿��ԭ������������йط�ӦΪ
C��s��+2NO��g��?N2��g��+CO2��g����H
��2L�����ܱ����м���������C��NO��Ӧ������ʵ���������
| ʵ���� | �¶�/��C | ��ʼʱNO�����ʵ���/mol | ƽ��ʱN2�����ʵ���/mol |
| 1 | 700 | 0.40 | 0.09 |
| 2 | 800 | 0.24 | 0.08 |
| 3 | 800 | 0.20 | a |
�ڽ�ϱ������ݣ��жϸ÷�Ӧ�ġ�H��0��������������������Ǽ���700��C��800��C��ƽ�ⳣ��K1��K2�����ԡ�H��0��
��ʵ��3�ﵽƽ��ʱ��NO��ת����Ϊ67%��������2λ��Ч���֣�
�ܸ���ʵ��2���ݣ����跴Ӧ��t1ʱ�̴ﵽƽ�⣬��t2ʱ�̱����¶Ȳ��䣬���������ѹ����ԭ��һ�룬����ͼ����������������CO2�����������CO2����ʱ��仯�����ߣ�
| A�� | �������ϲ˵Ⱥ�����ֲ���к��зḻ��I2 | |
| B�� | ���պ�����Ʒʱ������Ӧ�õ���ʯ�����ϼ��� | |
| C�� | ������CCl4���ѻ����͵��л��ܼ��ѵ���ȡ���� | |
| D�� | ��Һ©����Һ�����������ó�����������$\frac{2}{3}$ |
| A�� | п����ϡH2SO4��Ӧ | B�� | Ba��OH��2•8H2O��NH4Cl��Ӧ | ||
| C�� | ���ȵ�̼��CO2��Ӧ | D�� | ������������������ |
 l2��NaCl��H2O��CO2��д������ƽ�йػ�ѧ����ʽ�����������ת�Ƶķ������Ŀ
l2��NaCl��H2O��CO2��д������ƽ�йػ�ѧ����ʽ�����������ת�Ƶķ������Ŀ ��
��