��Ŀ����
����Ŀ��4����ױ���2����ױ��Լ�HBr������Ҫ���л��м��塣�Լױ���Һ�塢���ۡ�ˮΪԭ�������������ʵ�ʵ�鲽�����£�

����1����������ƿ��(װ����ͼ��ʾ)����0.3g���ۣ�46g�ױ���60 mLˮ���ڷ�Һ©���м���80 gҺ�壬�ټ���10 mLˮ��
����2����Һ©���μ�Һ�壬���Ͻ��豣�ַ�Ӧ�¶���20��25��֮�䡣���Һ©���е�ˮҲȫ������������ƿ�У���������0.5h�����ɷ�Ӧ��ȫ��
����3��������ˮԡװ�ã�����������ƿ����Ӧ�¶���122��126�棬���Һ�����ˮ���У���ˮ�����Ž��²�Һ���Լ���������ƿ�У��ϲ�Һ�嵹���ձ��С�
����4����������ƿ��Һ����д����������4����ױ���2����ױ������ߵ��ܲ���Ϊ51.3g��
�ش��������⣺
(1)ʵ����Һ��ĵμӷ�ʽ��__________���ڷ�Һ©���м���80gҺ�壬�ټ���10mLˮ��Ŀ����_________��
(2)����ˮӦ�������ܵ�________(a��b)�˽��룻ʵ���з��ַ�ˮ���е�Һ����ֻ�����Ӧ��ȡ������_____________________��
(3)д����Ӧװ��������4����ױ��Ļ�ѧ����ʽ��_________�������4����ױ���2����ױ���Ӧ���õIJ�����___________��
(4)��ʵ��װ���л�ȱ�ٵı�Ҫʵ��������____________��
(5)��Ӧ�¶ȱ�����20��25��֮�䣬��ԭ����__________��
(6)��ʵ���У�һ��ױ�(4����ױ���2����ױ�)�IJ���Ϊ_________��
���𰸡������μ� ��ֹ��Ļӷ� a ���²�Һ�弰ʱ�ų� ![]() +Br2
+Br2![]()
![]() +HBr ���� �¶ȼ� ������ͼױ��Ļӷ� 60.0��
+HBr ���� �¶ȼ� ������ͼױ��Ļӷ� 60.0��
��������
(1)Һ�����ӷ���
(2)����ˮ�½��ϳ�����ˮ���¶��пڣ�
(3)�÷�Ӧ�����ȷ����嵥�������ķ�Ӧ�����ɵ��廯��Ϊ����������Ԫ���غ���д����ʽ��������Է��뻥�ܵ��л��
(4)��ʵ����¶���Ҫ��
(5)�¶ȹ��߷�Ӧ��ӷ���
(6)����=![]() ��
��
(1)Һ���ӷ����ױ�����ķ�Ӧ���ȣ��μ�Һ�����ᵼ�²���Һ��δ��Ӧ�ͻӷ��ˣ�����ˮҲ���Է�ֹ��Һ©�����Һ��ӷ����ʴ�Ϊ�������μӣ���ֹ��Ļӷ���
(2)����ˮӦ��a�˽��룻ʵ���з��ַ�ˮ���е�Һ����ֻ�����Ӧ���²�Һ�弰ʱ�ų����ʴ�Ϊ��a�����²�Һ�弰ʱ�ų���
(3)�÷�Ӧ�������嵥�ʽ������������廯�����廯��������ʹ�ױ����嵥�ʷ���ȡ����Ӧ�������4����ױ���2����ױ���Ӧ���õIJ��������ʴ�Ϊ��![]() +Br2
+Br2![]()
![]() +HBr������
+HBr������
(4)��ʵ������Ҫ�����¶ȣ�������Ҫ�¶ȼƣ��ʴ�Ϊ���¶ȼƣ�
(5)�嵥�ʺͼױ����ӷ����¶ȹ���ӿ췴Ӧ��Ļӷ������������ʵͣ��ʴ�Ϊ��������ͼױ��Ļӷ���
(6)46g�ױ������ʵ���Ϊ![]() ��80gҺ������ʵ���Ϊ
��80gҺ������ʵ���Ϊ![]() �����ݷ�Ӧ����ʽ��֪�嵥�ʹ����������������������4����ױ���2����ױ��Ļ���ﹲ0.5mol������Ϊ171g/mol��0.5mol=85.5g�����Բ���Ϊ
�����ݷ�Ӧ����ʽ��֪�嵥�ʹ����������������������4����ױ���2����ױ��Ļ���ﹲ0.5mol������Ϊ171g/mol��0.5mol=85.5g�����Բ���Ϊ![]() =60%���ʴ�Ϊ��60%��
=60%���ʴ�Ϊ��60%��

����Ŀ���ߴ�̼����(MnCO3)�㷺Ӧ���ڵ�����ҵ����������ܴ��Բ��ϡ���ҵ���ú��̷�ˮ(��Ҫ��Mn2+��![]() ��H+��Fe2+��Al3+��Cu2+)�Ʊ�̼���̡���������淋Ĺ����������£�
��H+��Fe2+��Al3+��Cu2+)�Ʊ�̼���̡���������淋Ĺ����������£�

��֪ijЩ������ȫ������pHֵ�����
������ | Fe(OH)3 | Al(OH)3 | Cu(OH)2 | Mn(OH)2 | CuS | MnS | MnCO3 |
������ȫʱ��pH | 3.7 | 5.2 | 6.4 | 9.8 | ��0 | ��7 | ��7 |
�ش��������⣺
(1)�Լ�X��___________ (����)��
a.Cl2 b.MnO2 c.ŨHNO3 d.H2O2
(2)������1������Ҫ�ɷֵĻ�ѧʽΪ__________��
(3)�����ؽ�����ʱ������Ӧ�����ӷ���ʽ��______����Ӧ��ʹ��(NH4)2S����ʹ��Na2S��ԭ����________��
(4)��50��̼�����õ��ߴ�̼���̣���Ӧ�����ӷ���ʽΪ_____����Ӧ��ͨ��������Թ�����NH4HCO3���ҿ�����Һ��pHΪ6.8��7.4�������Թ�����NH4HCO3��Ŀ����______ʹMnCO3������ȫ����Һ��pH���ܹ��͵�ԭ����_____��
(5)�� MnCO3���Ƶ���Ҫ�Ĵ���MnO2��2MnCO3+O2=2MnO2+2CO2�����ڿ����м���460.0g��MnCO3���õ�332.0g��Ʒ������Ʒ������ֻ��MnO����ò�ƷMnO2������������___________��
����Ŀ��![]() �ȱ���
�ȱ���![]() ��Ҫ��������ũҩ���ݼ����������������ἰ�й�ҵ��ֵ�ĵͼ���������ͼΪʵ�����Ʊ�
��Ҫ��������ũҩ���ݼ����������������ἰ�й�ҵ��ֵ�ĵͼ���������ͼΪʵ�����Ʊ�![]() �ȱ����װ�á�
�ȱ����װ�á�
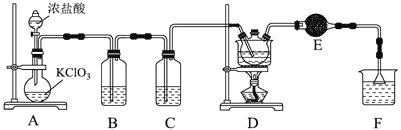
��֪��������ʵ������������±���ʾ��
���� | �۵� | �е� | �ܽ��� |
| 14 | 190 | ����ˮ���Ҵ����� |
���� |
| 141 | ����ˮ���Ҵ����� |
|
|
| ��ˮ���ҷ�Ӧ���������Ҵ� |
�Ʊ���������������ƿ�з���![]() �����
�����![]() ���Ȼ���
���Ȼ���![]() ������
������![]() ��������
��������![]() ������ͨ�������������¶���
������ͨ�������������¶���![]() ֮���Լ��Ӧ
֮���Լ��Ӧ![]() ��
��
�ش��������⣺
![]() װ���з�Ӧ�����ӷ���ʽΪ_____________________________________________��������
װ���з�Ӧ�����ӷ���ʽΪ_____________________________________________��������![]() ����״����ʱ��ת�Ƶ��ӵ���ĿΪ________________��
����״����ʱ��ת�Ƶ��ӵ���ĿΪ________________��
![]() ijͬѧ��������Dװ��������ȱ�ݣ��ֱ���_____________��________________��
ijͬѧ��������Dװ��������ȱ�ݣ��ֱ���_____________��________________��
![]() ���ʵ���ᴿ��Ʒ��_________________________________________________________��
���ʵ���ᴿ��Ʒ��_________________________________________________________��
![]() �ⶨ��Ʒ���ȡ�
�ⶨ��Ʒ���ȡ�
�����ȡ1.20g��Ʒ![]() ���ʲ���
���ʲ���![]() ����ƿ�У�����
����ƿ�У�����![]() ����������Һ���ȣ���ȴ�����¡�����
����������Һ���ȣ���ȴ�����¡�����![]() ���ᣬһ��ʱ�����ƿ�е���Һȫ��ת����
���ᣬһ��ʱ�����ƿ�е���Һȫ��ת����![]() ����ƿ�У���ˮ����
����ƿ�У���ˮ����![]() ��Һ��Ϊ�����
��Һ��Ϊ�����![]() ��
��
���������ƿ�и�ȡ![]() ��Һ����ƿ�У���
��Һ����ƿ�У���![]() ��ָʾ������
��ָʾ������![]() ��Һ�ֱ�ζ���Һ�е�
��Һ�ֱ�ζ���Һ�е�![]() ��֪��
��֪��![]() Ϊש��ɫ������������������
Ϊש��ɫ������������������![]() ��ƽ������ʵ�飬���õζ����������ʾ��
��ƽ������ʵ�飬���õζ����������ʾ��
ʵ����� ʵ������ | ��һ�� | �ڶ��� | ������ | |
| �ζ�ǰ | 0 |
|
|
��� |
|
|
| |
![]() ���������Ŀ����_______________________________________��
���������Ŀ����_______________________________________��
![]() ���������У��ﵽ�ζ��յ��������___________________________________________��
���������У��ﵽ�ζ��յ��������___________________________________________��
![]() ��Ʒ��
��Ʒ��![]() �ȱ������������Ϊ__________
�ȱ������������Ϊ__________![]() ������λ��Ч����
������λ��Ч����![]() ��
��

