��Ŀ����
�� NH4Cl��������ʵ�飬���жϷ�����������˵��ԭ��
(1) �ò�����պȡ���� NH4Cl��Һ��pH��ֽ�Ӵ�ʱ����ֽ��ʾ����ɫ�����ɫ����ȣ��ɷ��� NH4Cl��Һ��pH 7(�<������=����>�������� �ԣ���ᡱ��������С�������ԭ��������ӷ���ʽ��ʾΪ ��
(2)��NH4Cl��Һ�м���þ�ۣ��ɹ۲쵽���������ɣ�����Ҫ�ɷ�Ϊ ��
(3) 25��ʱ����0.1mol/L�İ�ˮ�м��������Ȼ�粒��壬�������ܽ�����ҺpH��С����Ҫԭ���ǣ�����ţ� ��
A����ˮ���Ȼ�立�����ѧ��Ӧ B���Ȼ����Һˮ�������ԣ�������c(H+)
C���Ȼ������ˮ���������������ӣ������˰�ˮ�ĵ��룬ʹc(OH�D)��С
(1) �ò�����պȡ���� NH4Cl��Һ��pH��ֽ�Ӵ�ʱ����ֽ��ʾ����ɫ�����ɫ����ȣ��ɷ��� NH4Cl��Һ��pH 7(�<������=����>�������� �ԣ���ᡱ��������С�������ԭ��������ӷ���ʽ��ʾΪ ��
(2)��NH4Cl��Һ�м���þ�ۣ��ɹ۲쵽���������ɣ�����Ҫ�ɷ�Ϊ ��
(3) 25��ʱ����0.1mol/L�İ�ˮ�м��������Ȼ�粒��壬�������ܽ�����ҺpH��С����Ҫԭ���ǣ�����ţ� ��
A����ˮ���Ȼ�立�����ѧ��Ӧ B���Ȼ����Һˮ�������ԣ�������c(H+)
C���Ȼ������ˮ���������������ӣ������˰�ˮ�ĵ��룬ʹc(OH�D)��С
��1���� �� NH4++H2O NH3��H2O+H+
NH3��H2O+H+
��1��H2 ��3��C
 NH3��H2O+H+
NH3��H2O+H+��1��H2 ��3��C
��1��NH4Cl��ǿ�������Σ�NH4��ˮ�������ԣ�����ʽΪNH4++H2O NH3��H2O+H+��
NH3��H2O+H+��
��2���Ȼ�������ԣ������ܺ�þ��Ӧ����������
��3����ˮ�д��ڵ���ƽ��NH3��H2O NH4����OH�������������Ȼ�粒��壬����NH4��Ũ�ȣ����ư�ˮ�ĵ��룬���Լ��Խ��ͣ�pH��С����ѡC��
NH4����OH�������������Ȼ�粒��壬����NH4��Ũ�ȣ����ư�ˮ�ĵ��룬���Լ��Խ��ͣ�pH��С����ѡC��
 NH3��H2O+H+��
NH3��H2O+H+����2���Ȼ�������ԣ������ܺ�þ��Ӧ����������
��3����ˮ�д��ڵ���ƽ��NH3��H2O
 NH4����OH�������������Ȼ�粒��壬����NH4��Ũ�ȣ����ư�ˮ�ĵ��룬���Լ��Խ��ͣ�pH��С����ѡC��
NH4����OH�������������Ȼ�粒��壬����NH4��Ũ�ȣ����ư�ˮ�ĵ��룬���Լ��Խ��ͣ�pH��С����ѡC��
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ


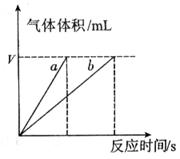
 �ֽⷴӦ���ʵ�Ӱ�죬ijͬѧ����֧�Թ��зֱ����
�ֽⷴӦ���ʵ�Ӱ�죬ijͬѧ����֧�Թ��зֱ����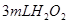 ��Һ���������ռ�
��Һ���������ռ�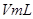 ���������ʱ�䣬ʵ���¼���£�
���������ʱ�䣬ʵ���¼���£�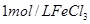
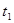
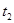
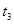
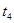
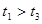 ��ԭ����__________��
��ԭ����__________��