��Ŀ����
��14�֣�
�����£���֪HCl��Һ��NaOH��Һ�����ζ���������ͼ��ʾ��

��1����һ������NaOH��Һ�еμ�HCl��Һ������Ϊͼ�� ���ʵ�ߡ������ߡ�����

��2����ͼΪ��10mLһ�����ʵ���Ũ�ȵ�NaHSO4��ҺX��һ�����ʵ���Ũ�ȵ�����������ҺY�ζ���ͼ������ͼ���Ƴ�X��Y�����ʵ���Ũ�ȷֱ��� �� ��
��3��ij�о���ѧϰС����о����⣺ʳ������������g/100mL���IJⶨ�����ǽ������µζ�������
A��ȡijƷ�ư״�25.00mL���� �����������ƣ��У�������ˮϡ��10����
B���� �����������ƣ���ȡϡ�ͺ�İ״���Һ20.00mL������250mL��ƿ�У����� ����ָʾ�����ƣ�1��2�Ρ�
C����0.05 mol��L?1NaOH����Һ�ζ������յ㡣���³�ʼ���յ������
��ע�⣺�ζ��ظ�����3�Ρ���
�������ϲ�������������ش��������⡣
�ٲ�����C���У��ζ�ʱ������ע�� ���յ������� ��
�������������в�������ʹ�ⶨ���ƫ�ߵ��� ��
a��ϡ�Ͱ״�����ˮԤ��δ������д���
b��ʢNaOH��Һ�ļ�ʽ�ζ���δ�ñ�Һ��ϴ
c���ζ�ǰ������ȷ���ζ��յ�ʱ���Ӷ���
d���ӽ��յ�ʱ������������ˮϴ����ƿ
�������С���������Ʒ���̱�����ע��һ�£����ܵ�ԭ��֮һ�� ��
�����£���֪HCl��Һ��NaOH��Һ�����ζ���������ͼ��ʾ��

��1����һ������NaOH��Һ�еμ�HCl��Һ������Ϊͼ�� ���ʵ�ߡ������ߡ�����

��2����ͼΪ��10mLһ�����ʵ���Ũ�ȵ�NaHSO4��ҺX��һ�����ʵ���Ũ�ȵ�����������ҺY�ζ���ͼ������ͼ���Ƴ�X��Y�����ʵ���Ũ�ȷֱ��� �� ��
��3��ij�о���ѧϰС����о����⣺ʳ������������g/100mL���IJⶨ�����ǽ������µζ�������
A��ȡijƷ�ư״�25.00mL���� �����������ƣ��У�������ˮϡ��10����
B���� �����������ƣ���ȡϡ�ͺ�İ״���Һ20.00mL������250mL��ƿ�У����� ����ָʾ�����ƣ�1��2�Ρ�
C����0.05 mol��L?1NaOH����Һ�ζ������յ㡣���³�ʼ���յ������
��ע�⣺�ζ��ظ�����3�Ρ���
�������ϲ�������������ش��������⡣
�ٲ�����C���У��ζ�ʱ������ע�� ���յ������� ��
�������������в�������ʹ�ⶨ���ƫ�ߵ��� ��
a��ϡ�Ͱ״�����ˮԤ��δ������д���
b��ʢNaOH��Һ�ļ�ʽ�ζ���δ�ñ�Һ��ϴ
c���ζ�ǰ������ȷ���ζ��յ�ʱ���Ӷ���
d���ӽ��յ�ʱ������������ˮϴ����ƿ
�������С���������Ʒ���̱�����ע��һ�£����ܵ�ԭ��֮һ�� ��
��14�֣�
��1������ ��2�֣� ��2��0.09 mol��L?1 0.03 mol��L?1 ��ÿ��2�֣�
��3����A��250mL����ƿ��1�֣�
��B����ʽ�ζ��ܣ�1�֣�����̪��Һ��1�֣�
����ƿ����Һ��ɫ�ı仯����1�֣�
��Һ����ɫ��Ϊdz��ɫ������30s�ڲ���ɫ����1�֣�
��a��b��2�֣�
��ʳ��ϡ��ʱ��������Ʋ�����ʵ��ϡ�ͱ��������������ͱ�����������ȡ���ƫС���ζ��յ���ɫ����30s��ȥ���ζ��������ڼ�ʽ�ζ��ܵļ��촦�����ݣ������̼�ˮ����ϡ�͵ȡ���1�֣�
��1������ ��2�֣� ��2��0.09 mol��L?1 0.03 mol��L?1 ��ÿ��2�֣�
��3����A��250mL����ƿ��1�֣�
��B����ʽ�ζ��ܣ�1�֣�����̪��Һ��1�֣�
����ƿ����Һ��ɫ�ı仯����1�֣�
��Һ����ɫ��Ϊdz��ɫ������30s�ڲ���ɫ����1�֣�
��a��b��2�֣�
��ʳ��ϡ��ʱ��������Ʋ�����ʵ��ϡ�ͱ��������������ͱ�����������ȡ���ƫС���ζ��յ���ɫ����30s��ȥ���ζ��������ڼ�ʽ�ζ��ܵļ��촦�����ݣ������̼�ˮ����ϡ�͵ȡ���1�֣�
��������к͵ζ�
��1����һ������NaOH��Һ�еμ�HCl��Һʱ�������Һ��pH��С���ʷ�������
��2����X��Y�����ʵ���Ũ�ȷֱ�CX��CY������ͼʾ�ɵõ���������ʽ��
0.01CX=0.03CY 0.01=��0.01CX��0.02CY��/0.03
��ã�CX=0.09 mol��L?1��CY=0.03 mol��L?1
��3��A����ȷ�����ơ�ϡ����Һ������Ϊ����ƿ
B����ȷ��ȡ����Һ��ɲ�����ʽ�ζ��ܣ�������ǿ��ζ����ᣬ�к�ʱ��Һˮ��ʼ��ԣ���ò��ñ�ɫ��ΧΪ���������ָʾ��������̪
C�������ζ�ʱ�������ջ�����������ת��ƿ������ע����ƿ����Һ��ɫ�ı仯��
�յ���������Һ����ɫ��Ϊdz��ɫ������30s�ڲ���ɫ
��a������ˮ����ʱһ�����������γ��������ʣ��ʽ����ĵļ�Һƫ�࣬�ⶨ���ƫ��
b��ʢNaOH��Һ�ļ�ʽ�ζ���δ�ñ�Һ��ϴ��ʹ��Һ��Ũ��ƫС����Һ�����ƫ�ⶨ���ƫ��
c���ζ�ǰ������ȷ���ζ��յ�ʱ���Ӷ�������ʹ��Һ�����ƫС���ⶨ���ƫ��
d����Ӱ��
�������̼�ˮ����ϡ�͵�
��1����һ������NaOH��Һ�еμ�HCl��Һʱ�������Һ��pH��С���ʷ�������
��2����X��Y�����ʵ���Ũ�ȷֱ�CX��CY������ͼʾ�ɵõ���������ʽ��
0.01CX=0.03CY 0.01=��0.01CX��0.02CY��/0.03
��ã�CX=0.09 mol��L?1��CY=0.03 mol��L?1
��3��A����ȷ�����ơ�ϡ����Һ������Ϊ����ƿ
B����ȷ��ȡ����Һ��ɲ�����ʽ�ζ��ܣ�������ǿ��ζ����ᣬ�к�ʱ��Һˮ��ʼ��ԣ���ò��ñ�ɫ��ΧΪ���������ָʾ��������̪
C�������ζ�ʱ�������ջ�����������ת��ƿ������ע����ƿ����Һ��ɫ�ı仯��
�յ���������Һ����ɫ��Ϊdz��ɫ������30s�ڲ���ɫ
��a������ˮ����ʱһ�����������γ��������ʣ��ʽ����ĵļ�Һƫ�࣬�ⶨ���ƫ��
b��ʢNaOH��Һ�ļ�ʽ�ζ���δ�ñ�Һ��ϴ��ʹ��Һ��Ũ��ƫС����Һ�����ƫ�ⶨ���ƫ��
c���ζ�ǰ������ȷ���ζ��յ�ʱ���Ӷ�������ʹ��Һ�����ƫС���ⶨ���ƫ��
d����Ӱ��
�������̼�ˮ����ϡ�͵�

��ϰ��ϵ�д�
�����Ŀ
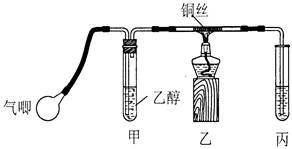
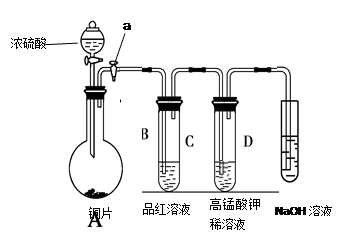
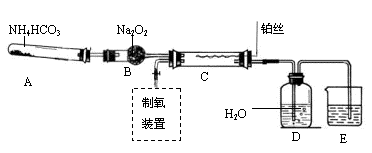
 l3��Һ�ܽ�Ag�ķ�Ӧ�������ȫ���������ԭ����������������������������
l3��Һ�ܽ�Ag�ķ�Ӧ�������ȫ���������ԭ����������������������������
 _________________(2��).
_________________(2��).
 ��ѧ����ʽ��__________________________��
��ѧ����ʽ��__________________________�� ____________��
____________�� __�������
__�������