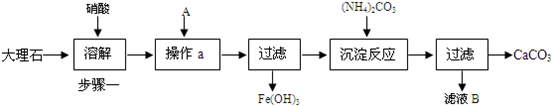
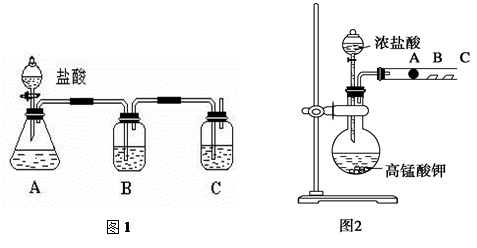
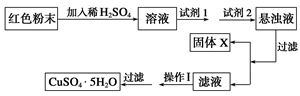
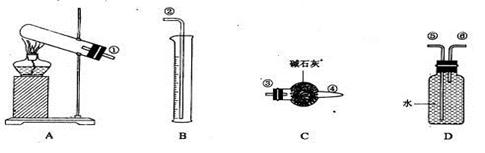
��Ŀ����
��6�֣�Ϊ���о����������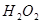 �ֽⷴӦ���ʵ�Ӱ�죬ijͬѧ����֧�Թ��зֱ����
�ֽⷴӦ���ʵ�Ӱ�죬ijͬѧ����֧�Թ��зֱ����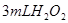 ��Һ���������ռ�
��Һ���������ռ�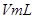 ���������ʱ�䣬ʵ���¼���£�
���������ʱ�䣬ʵ���¼���£�
��ش�
��1����������ֽ�Ļ�ѧ����ʽ��__________��
��2��ʵ��٢����о�__________�Է�Ӧ���ʵ�Ӱ�졣
��3��ʵ����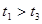 ��ԭ����__________��
��ԭ����__________��
��4��ʵ��٢ܵIJⶨ��������ͼ������a��Ӧ��ʵ�������__________����١��ܡ�����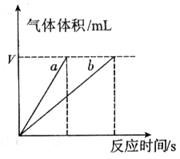
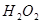 �ֽⷴӦ���ʵ�Ӱ�죬ijͬѧ����֧�Թ��зֱ����
�ֽⷴӦ���ʵ�Ӱ�죬ijͬѧ����֧�Թ��зֱ����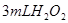 ��Һ���������ռ�
��Һ���������ռ�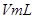 ���������ʱ�䣬ʵ���¼���£�
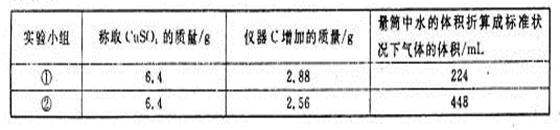
���������ʱ�䣬ʵ���¼���£�| ʵ����� | 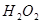 ��ҺŨ�� ��ҺŨ�� | 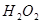 ��Һ�¶� ��Һ�¶� | ���� | ����ʱ�� |
| �� | 5% | 20�� | 2�� |  |
| �� | 5% | 40�� | 2��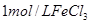 | 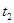 |
| �� | 10% | 20�� | 2��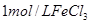 | 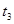 |
| �� | 5% | 20�� | ��ʹ�� |  |
��1����������ֽ�Ļ�ѧ����ʽ��__________��
��2��ʵ��٢����о�__________�Է�Ӧ���ʵ�Ӱ�졣
��3��ʵ����
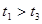 ��ԭ����__________��
��ԭ����__________����4��ʵ��٢ܵIJⶨ��������ͼ������a��Ӧ��ʵ�������__________����١��ܡ�����

��1��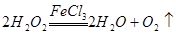 ��2�֣�д
��2�֣�д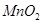 �������д�������۷֣�
�������д�������۷֣�
��2���¶ȣ�1�֣�
��3��������������ͬʱ��1�֣���ʵ��۵ķ�Ӧ��Ũ�ȴ���ʵ��ٵķ�Ӧ��Ũ�ȣ�����ʵ��۵ķ�Ӧ���ʴ���ʵ��ٵķ�Ӧ���ʣ�1�֣�
��4���٣�1�֣�
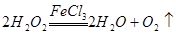 ��2�֣�д
��2�֣�д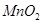 �������д�������۷֣�
�������д�������۷֣���2���¶ȣ�1�֣�
��3��������������ͬʱ��1�֣���ʵ��۵ķ�Ӧ��Ũ�ȴ���ʵ��ٵķ�Ӧ��Ũ�ȣ�����ʵ��۵ķ�Ӧ���ʴ���ʵ��ٵķ�Ӧ���ʣ�1�֣�
��4���٣�1�֣�
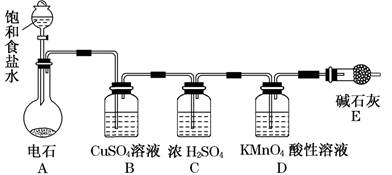
��1�����������ڴ����������£��ֽ�����������ˮ������ʽΪ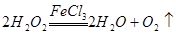 ��
��
��2��ʵ��٢���ֻ���¶��Dz�ͬ�ģ�ʵ���о������¶ȶԷ�Ӧ���ʵ�Ӱ�졣
��3��ʵ��٢���ֻ�з�Ӧ���Ũ���Dz�ͬ���ȣ�����������������ͬʱ��ʵ��۵ķ�Ӧ��Ũ�ȴ���ʵ��ٵķ�Ӧ��Ũ�ȣ�����ʵ��۵ķ�Ӧ���ʴ���ʵ��ٵķ�Ӧ���ʣ�����ʱ����١�
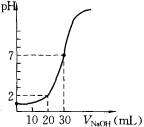
��4�������ܼӿ췴Ӧ���ʣ����ø���ͼ���֪�����߲���ʵ��ٵġ�
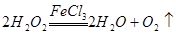 ��
����2��ʵ��٢���ֻ���¶��Dz�ͬ�ģ�ʵ���о������¶ȶԷ�Ӧ���ʵ�Ӱ�졣
��3��ʵ��٢���ֻ�з�Ӧ���Ũ���Dz�ͬ���ȣ�����������������ͬʱ��ʵ��۵ķ�Ӧ��Ũ�ȴ���ʵ��ٵķ�Ӧ��Ũ�ȣ�����ʵ��۵ķ�Ӧ���ʴ���ʵ��ٵķ�Ӧ���ʣ�����ʱ����١�
��4�������ܼӿ췴Ӧ���ʣ����ø���ͼ���֪�����߲���ʵ��ٵġ�

��ϰ��ϵ�д�
�����Ŀ