��Ŀ����
4��������Ϊ��ɫ���壬�۵�153.0��153.10C�������ھƾ�����Ҫ����������������ĭ���ϼ��������ܼ��ȣ���;ʮ�ֹ㷺������ԭ����ͼ1��
��ɫ�ϳ��������£�
����100mL������ƿ�м���2.00g�������������ƺͲ���ϳɣ���34.0mL30%��H2O2��Һ�ʹ��ӣ������½���15��20min����8.20g����ϩ�����Ӻ�װ�ã���ͼ2��ʾ���̶��г���������ȥ�����������پ��ҽ��貢���ȣ���Ӧ1.5h�õ��ȵĺϳ�Һ��
�ڰ��ȵĺϳ�Һ��һ���¶�����ȴ�����˵õ������ᾧ�壬���������ܽ�����¶ȱ仯�ϴ�����ϩ�Ľ�����¶ȱ仯����
�����ؽᾧ���ᴿ���壬ϴ�ӡ���������ò�Ʒ������Ϊ12.41g��
�ش��������⣺
��1����ͳ�����������ɫ����H2O2�ڷ�Ӧ�������������ã�����ɫ�Ʊ���ԭ����ȣ���ͳ�Ʒ�����̭����Ҫԭ���ǣ�Ũ�������ǿ��ʴ�ԣ����豸��ʴ���أ���Ӧ�л������ж��ĵ��������Ⱦ������
��2����ɫ��������a������Ϊ���������ܣ����������������������ԭ�ϵ������ʣ�
��3����ɫ�ϳ�ʱ������ͱ�����پ��ҽ����ԭ���ǻ���ϩ������ˮ�����ٽ��������ڷ�Ӧ���ֽӴ����ӿ췴Ӧ���ʣ�
��4������δ��Ӧ�Ļ���ϩ�ķ����Ƿ�Һ�����õ���Ҫ����������Ϊ��Һ©����
��5��ϴ�Ӿ���ʱӦѡ��ĺ��ʵ�ϴ�Ӽ�ΪB ������ĸ��ţ���
A������ˮ B����ˮ����� C���Ҵ� D����Һ
��6����ʵ���У�������IJ���Ϊ85%��
���� ��1����Ӧ�����Ὣ����������Ϊ�����ᡢ�������⽫����ϩ����Ϊ�����Ũ�������ǿ��ʴ�ԣ���Ӧ�л������ж��ĵ��������Ⱦ������
��2������aΪ���������ܣ��л����ӷ�����������ͨ������ˮ��ʹ�ӷ����л�������������������ԭ�������ʣ�
��3������ϩ������ˮ�����ٽ��������ڷ�Ӧ���ֽӴ����ӿ췴Ӧ���ʣ�
��4������ϩ������ˮ����ȡ��Һ�������з�����գ�
��5�������������ھƾ����ܽ�����¶ȱ仯�ϴ�Ҫ��С��ϴ�ӵ��µ���ʧ��
��6�����ݻ���ϩ���������㼺��������۲���������=��ʵ�ʲ��������۲�������100%��
��� �⣺��1����Ӧ�����Ὣ����������Ϊ�����ᡢ�������⽫����ϩ����Ϊ�����ᣬ�������������ã�����ɫ�Ʊ���ȣ�Ũ�������ǿ��ʴ�ԣ����豸��ʴ���أ���Ӧ�л������ж��ĵ��������Ⱦ������
�ʴ�Ϊ����������Ũ�������ǿ��ʴ�ԣ����豸��ʴ���أ���Ӧ�л������ж��ĵ��������Ⱦ������
��2���������ṹ������֪������aΪ���������ܣ������л����ӷ�����������ͨ������ˮ��ʹ�ӷ����л�������������������ԭ�ϵ������ʣ�
�ʴ�Ϊ�����������ܣ��������������ԭ�ϵ������ʣ�
��3�����ڻ���ϩ������ˮ�����ٽ��������ڷ�Ӧ���ֽӴ����ӿ췴Ӧ���ʣ��ϳ�ʱ������پ��ҽ��裬
�ʴ�Ϊ������ϩ������ˮ�����ٽ��������ڷ�Ӧ���ֽӴ����ӿ췴Ӧ���ʣ�
��4�����ڻ���ϩ������ˮ�����Բ�ȡ��Һ�������з�����գ����õ���Ҫ����Ϊ��Һ©����
�ʴ�Ϊ����Һ����Һ©����
��5�������������ھƾ����þƾ�ϴ�ӻ��ܽ����ʧ�ϴ����ܽ�����¶ȱ仯�ϴ��ñ�ˮ�����ϴ�ӣ����Լ�С��ϴ�ӵ��µ���ʧ��
�ʴ�Ϊ��B��
��6������������۲���Ϊ$\frac{8.20g}{82g/mol}$��146g/mol=14.6g���ʼ�����IJ���=��12.41g��14.6g����100%=85%��
�ʴ�Ϊ��85%��
���� ���⿼���л�����Ʊ�ʵ�飬�漰�Է�Ӧԭ���ķ�����������ʶ�����ʷ����ᴿ���Բ�����װ�õķ������ۡ����ʼ���ȣ��Ѷ��еȣ�

 ��ѧ�ҽ��������Ƴ�һ������ϸ��ȼ�ϵ�أ���������Ϊȼ�ϵ�����ȼ�ϵ�ؽṹʾ��ͼ��ͼ��ʾ�����ڸõ�ص�������ȷ���ǣ�������
��ѧ�ҽ��������Ƴ�һ������ϸ��ȼ�ϵ�أ���������Ϊȼ�ϵ�����ȼ�ϵ�ؽṹʾ��ͼ��ͼ��ʾ�����ڸõ�ص�������ȷ���ǣ�������| A�� | �õ�صĸ�����ӦΪO2+2H2O+4e-=4OH- | |
| B�� | ��صĸ�����ӦΪ��C6H12O6+6H2O=6CO2��+24H++24e- | |
| C�� | �ŵ�����У�H+������������Ǩ�� | |
| D�� | �ڵ�ط�Ӧ�У�ÿ����1mol�����������������ɱ�״����CO2����22.4L |

| A�� | ͼ1װ�ÿ���ȡ���ռ����﴿����NH3 | |
| B�� | ͼ2װ�ÿ�����ɡ���Ȫ��ʵ�� | |
| C�� | ͼ3װ�ÿɲ���Cu ��Ũ���ᷴӦ������������ | |
| D�� | ͼ4װ�ÿ��ñȽ�NaHCO3��Na2CO3�����ȶ��� |
| A�� | ���ʵ��۵㡢�е�ߵ� | |
| B�� | �ǽ���֮�䷢�����û���Ӧ | |
| C�� | �ǽ���������������̬�⻯������׳̶� | |
| D�� | �ǽ���Ԫ�ص�����������ˮ���������ǿ�� |
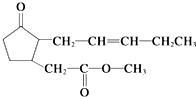 �������������ǿ�Ҷ��־õ������㣬�㷺�����˹����Ƶ��������У���ṹ��ʽ��ͼ��ʾ�����й��������������˵���в���ȷ���ǣ�������
�������������ǿ�Ҷ��־õ������㣬�㷺�����˹����Ƶ��������У���ṹ��ʽ��ͼ��ʾ�����й��������������˵���в���ȷ���ǣ�������| A�� | ����ʽΪC13H20O3 | B�� | ���������������ˮ | ||
| C�� | ��ʹ����KMnO4��Һ��ɫ | D�� | ��ʹBr2��CCl4��Һ��ɫ |
| A�� | �ڲⶨ�кͷ�Ӧ�ķ�Ӧ��ʵ���У�Ҫ��ȡ����¶� | |
| B�� | �к͵ζ�ʵ���У�ϴ�������ƿ����Ҫ���� | |
| C�� | ��CH3COONa��Һ�е���ʯ����Һ����Һ���� | |
| D�� | ���ɫZnS�����ϵμ�CuSO4��Һ��������Ϊ��ɫ��˵��Ksp��ZnS����Ksp��CuS�� |
 ��ӦN2O4��g��?2NO2��g����H=+57kJ•mol-1�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ���ǣ�������
��ӦN2O4��g��?2NO2��g����H=+57kJ•mol-1�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ���ǣ�������| A�� | A��C����ķ�Ӧ���ʣ�A��C | |
| B�� | A��B�����������ɫ��Adz��B�� | |
| C�� | A��C���������ƽ����Է���������A��C | |
| D�� | ��״̬B��״̬A�������ü��ȵķ��� |
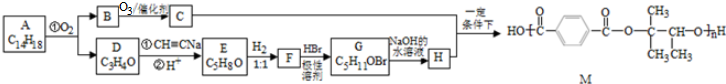
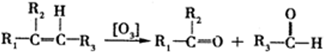
 ��D�Ļ�ѧ�����DZ�ͪ��
��D�Ļ�ѧ�����DZ�ͪ��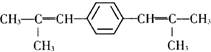 ��
�� ��
��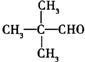 ����ṹ��ʽ����
����ṹ��ʽ����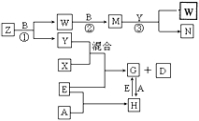 ��2����Ӧ���б������������뱻��ԭ�����ʵ����ʵ���֮��Ϊ2��1
��2����Ӧ���б������������뱻��ԭ�����ʵ����ʵ���֮��Ϊ2��1