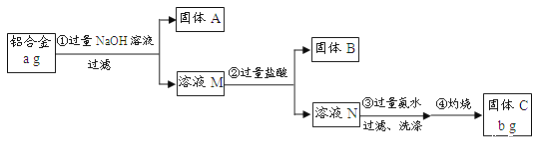
��Ŀ����
����Ŀ�������£���a mol N2��b mol H2�Ļ������ͨ��һ���̶��ݻ����ܱ������У��������·�Ӧ��N2 (g) �� 3 H2(g) ![]() 2NH3(g)��
2NH3(g)��
��1������Ӧijʱ��tʱ��n t (N2) = 13 mol��n t (NH3) = 6 mol����a =____mol��
��2����Ӧ��ƽ��ʱ�������������Ϊ716.8 L������£�������NH3�ĺ���(�������)Ϊ25%��ƽ��ʱNH3�����ʵ���_____��
��3��ԭ���������ƽ��������������ʵ���֮�ȣ�д����������ȡ���ͬ����n(ʼ)��n(ƽ) =______��
��4��ԭ��������У�a��b =_____��
��5���ﵽƽ��ʱ��N2��H2��ת����֮�ȣ���(N2)�æ�(H2)= ______��
��6��ƽ���������У�n(N2)��n(H2)��n(NH3) =______��
���𰸡�16 mol 8 mol 5��4 2��3 1��2 3��3��2
��������
��1�����ݻ�ѧ����ʽ����aֵ��
��2����Ӧ��ƽ��ʱ�������������Ϊ716.8 L������£�������������ʵ�����![]() 32mol������NH3�ĺ���(�������)Ϊ25%���㰱�������ʵ�����
32mol������NH3�ĺ���(�������)Ϊ25%���㰱�������ʵ�����
��3�����ò���������ԭ�����������ʵ�����
��4�����ݣ�1����֪a=16�����ݣ�3����֪ԭ�����������ʵ�����40mol��
��5�����á�����ʽ������N2��H2��ת����֮�ȣ�
��6�����ݡ�����ʽ���ж�ƽ���������и���������ʵ����ȣ�
��1������N2 (g)�� 3H2(g)![]() 2NH3(g)��Ӧ����֪����Ӧijʱ��tʱ��n (NH3) = 6 mol��������n (N2) =3 mol����ʼ����n (N2)= 13mol+ 3mol =16mol��
2NH3(g)��Ӧ����֪����Ӧijʱ��tʱ��n (NH3) = 6 mol��������n (N2) =3 mol����ʼ����n (N2)= 13mol+ 3mol =16mol��
��2����Ӧ��ƽ��ʱ�������������Ϊ716.8 L������£�������������ʵ�����![]() 32mol��NH3�ĺ���(�������)Ϊ25%�������������ʵ�����32mol��25%=8mol��
32mol��NH3�ĺ���(�������)Ϊ25%�������������ʵ�����32mol��25%=8mol��
��3���跴Ӧ���������ʵ�������n��
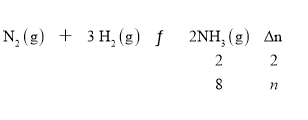
n=![]() 8mol������ԭ�����������ʵ�����32mol+8mol=40mol��ԭ���������ƽ��������������ʵ���֮��40:32=5:4��
8mol������ԭ�����������ʵ�����32mol+8mol=40mol��ԭ���������ƽ��������������ʵ���֮��40:32=5:4��
��4�����ݣ�1����֪a=16�����ݣ�3����֪ԭ�����������ʵ�����40mol������b=40mol-16mol=24mol��a��b =16:24=2:3��
��5��
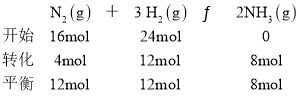
N2��ת����Ϊ![]() ��H2��ת����Ϊ
��H2��ת����Ϊ![]() ��������(N2)����(H2)=1:2��
��������(N2)����(H2)=1:2��
��5������
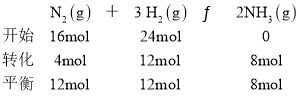
ƽ���������У�n(N2)��n(H2)��n(NH3) =12:12:8=3:3:2��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ�����в���ǰ36��Ԫ�ص����ʻ�ԭ�ӽṹ���±�
Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
R | ��̬ԭ�ӵ��������3��δ�ɶԵ��ӣ��������2������ |
S | ��������ˮ���ҷ�Ӧ��������Һ�������� |
T | ��̬ԭ��3d�������1������ |
X | �� |
��1��RԪ�صĵ�һ������Ҫ������ͬ�������ڵ�Ԫ�أ�ԭ����________________________________________________________��
��2��SԪ�صĻ��ϼ��Ƿ������ۣ�__________��ԭ����__________________________________�����������Ų�ʽΪ________________________��
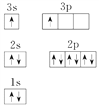
��3��TԪ�ص�ԭ��N�ܲ��ϵ�����Ϊ__________����ԭ�ӽṹʾ��ͼΪ__________��
��4��X�ĺ�������Ų�ͼΥ����__________����X���ʡ�������μ����������εȿ����������ȼ��ʱ�����������ɫ�Ĺ⣬����ԭ�ӽṹ��֪ʶ���ͷ����ԭ��____________________________________________________________________��
���𰸡� ��ԭ��2p���������������ͣ��ȶ� �� F�ĵ縺�����ֻ�ܵõ��� 2s22p5 2  �������ԭ�� ���Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ���Թ����ʽ�ͷ�����
�������ԭ�� ���Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ���Թ����ʽ�ͷ�����
�����������������RԪ�صĻ�̬ԭ���������3��δ�ɶԵ��ӣ��������2�����ӣ�R��NԪ�أ�SԪ�صĵ�������ˮ���ҷ�Ӧ��������Һ�������ԣ�S��FԪ�أ�TԪ�صĻ�̬ԭ��3d�������1�����ӣ�T��21��Ԫ��Sc�� XԪ�ص�ԭ�Ӻ�����12�����ӣ�X��MgԪ�ء�
�������������Ϸ�������1��R��NԪ������ԭ��2p���������������ͣ��ȶ�,���Ե�һ������Ҫ������ͬ�������ڵ�OԪ����
��2��Ԫ��F�ĵ縺�����ֻ�ܵõ���������FԪ��û�����ۣ�FԪ�ص����������Ų�ʽΪ2s22p5��
��3��Scԭ�ӵĺ�������Ų�ʽ��1s22s22p63s23p63d14s2������N�ܲ��ϵ�����Ϊ2����ԭ�ӽṹʾ��ͼΪ ����4�������������ԭ����Mgԭ�������2������Ӧ�Ų���3s����������Ժ�������Ų�ͼΥ�����������ԭ�������Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ���Թ����ʽ�ͷ�����������ȼ�����ʱ�����������ɫ�Ĺ���
����4�������������ԭ����Mgԭ�������2������Ӧ�Ų���3s����������Ժ�������Ų�ͼΥ�����������ԭ�������Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ���Թ����ʽ�ͷ�����������ȼ�����ʱ�����������ɫ�Ĺ���
�����͡�������
��������
20
����Ŀ�����в��ֶ�����Ԫ�ص����ʻ�ԭ�ӽṹ���±���
Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
T | ��������ˮ���ҷ�Ӧ��������Һ�������� |
X | L��p��������s��������2�� |
Y | ��������Ԫ�صļ������а뾶��С |
Z | L��������δ�ɶԵ��� |
��1��д��Ԫ��X�����ӽṹʾ��ͼ__________��
��2��д��YԪ������������ˮ����ֱ���HCl��NaOH��Һ��Ӧ�����ӷ���ʽ_______________________��_________________________��
��3��д��Z��Y�ĵ����Ų�ʽ______________��________________��
��4��Ԫ��T����Ԫ����ȣ��ǽ����Խ�ǿ����__________(��Ԫ�ط��ű�ʾ)�����б�������֤����һ��ʵ����__________��
A����̬�⻯��Ļӷ��Ժ��ȶ���
B�����ʷ����еļ���
C����Ԫ�صĵ縺��
D�������������
E���⻯����X��H���ļ���(X����T��Cl����Ԫ��)
F������������Ȼ���еĴ�����ʽ
��5��̽Ѱ���ʵ����ʲ�������ѧϰ����Ҫ����֮һ��T��X��Y��Z����Ԫ�صĵ����л�ѧ�������Բ�ͬ���������ֵ��ʵ���__________(��Ԫ�ط���)��������________________________________________________��