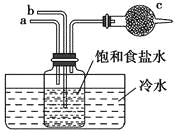
��Ŀ����
����Ŀ��ʵ������Ҫ����0.50mol/LNaCl��Һ480mL����ʹ��NaCl�������ƣ������в������������ʵ������֣���ʹ��������������
��1��ѡ����������ɱ�ʵ��������������У�������ƽ����ȷ��0.1 g����ҩ�ס��ձ�����������______��_______�Լ�����������ƬֽƬ��
��2�����㡣���Ƹ���Һ��ȡNaCl����_______g��
��3�����ù��̡�
����ƽ���㡣
�ڳ���������NaCl����Ӧ������ƽ��_______��������������������������
�۳�����ϣ���ҩƷ�����ձ��С�
���ܽ⡢��ȴ���ò�ʵ������Ҫʹ�ò�������Ŀ����________��
��ת�ơ�ϴ�ӡ���ת��ʱӦʹ��_____��������Ҫϴ���ձ�2-3����Ϊ��______��
���ݡ�ҡ�ȡ�
�߽���õ���Һ����һ��ʱ�����ָ�����Լ�ƿ�������ñ�ǩ��ע�����Ƶ�ʱ�䡢��Һ���Ƽ�Ũ�ȡ�
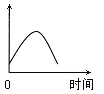
��4�������ƹ����У�ijѧ���۲춨��ʱҺ�������ͼ��ʾ��������Һ��Ũ�Ȼ�______����ߡ�����ƫ�͡�����Ӱ�족����
���𰸡�500 mL����ƿ ��ͷ�ι� 14.6 ���� ���裬����NaCl�ܽ� ������ ��֤����ȫ��ת�Ƶ�����ƿ�� ƫ��
��������
���⿼������0.50mol/LNaCl��Һ480mL������������ƿ��ѡ�����Ʋ����е�ע�������������
��1������480ml��Һ��Ҫѡ��500mL����ƿ������Ҫ����0.50mol/L 480mL NaCl��Һ����Ҫ��ʵ�������ǣ�������ƽ����ȷ��0.1 g����ҩ�ס��ձ�������������ͷ�ιܡ�500 mL����ƿ��
��2����Ϊѡ���500 mL����ƿ ��������Ҫ�����Ȼ��Ƶ������ǣ�0.50mol/L��0.5L��58.5g/mol=14.6��
��3���ڳ��������У�����̫ƽ�����̷���Ҫ��������Ʒ�����̷����룻
���ܽ�����У�Ϊ�˼ӿ��Ȼ����ܽ⣬ʹ�ò��������裻
�ݰ��ձ����ܽ���Ȼ���ת�Ƶ�����ƿ�У���ʱ���ò������������ձ������ܽ���Ȼ���ת�Ƶ�����ƿ���ձ��ڱڻ�մ���Ȼ��ƣ�Ϊ�˼�Сʵ������ʱ����Ҫϴ���ձ������DZ�֤����ȫ��ת�Ƶ�����ƿ�У�
��4�������ƹ����У�����ʱ����Һ�棬��ʱ������Һ�����ƫ�������Ȼ�����Һ��Ũ�Ȼ�ƫ�͡�

����Ŀ����Ȼ������Ҫ�ɷ�ΪCH4��һ�㻹����C2H6�����࣬����Ҫ��ȼ�Ϻͻ���ԭ�ϡ�
(1)������һ�������ɷ������·�Ӧ��C2H6(g)= C2H4(g)+H2(g) ��H��������ʵ�ȼ�����������±���ʾ��
���� | C2H6(g) | C2H4(g) | H2(g) |
ȼ������H/( kJ��mol1) | -1560 | -1411 | -286 |
����H=_________kJ��mol1��
����߸÷�Ӧƽ��ת���ʵķ�����_________��_________��
��������ͨ������ʵ�����������������ڵ�ѹ��(p)����������Ӧ�������ƽ��ת����Ϊ������Ӧ��ƽ�ⳣ��Kp=_________(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ=��ѹ�����ʵ�������)��
(2)�����£�������������ķ�Ӧ���£�2CH4![]() C2H6+H2����Ӧ�ڳ��ڽε����ʷ���Ϊ��r=k��
C2H6+H2����Ӧ�ڳ��ڽε����ʷ���Ϊ��r=k��![]() ������kΪ��Ӧ���ʳ�����
������kΪ��Ӧ���ʳ�����
���跴Ӧ��ʼʱ�ķ�Ӧ����Ϊr1�������ת����Ϊ��ʱ�ķ�Ӧ����Ϊr2����r2=_____ r1��
�ڶ��ڴ��ڳ��ڽεĸ÷�Ӧ������˵����ȷ����_________��
A�����Ӽ���Ũ�ȣ�r���� B������H2Ũ�ȣ�r����
C��������������������� D�����ͷ�Ӧ�¶ȣ�k��С
(3)CH4��CO2���DZȽ��ȶ��ķ��ӣ���ѧ�����õ绯ѧװ��ʵ�����ַ��ӵ����ת������ԭ������ͼ��ʾ��
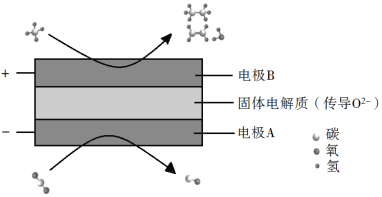
�������ϵķ�ӦʽΪ_________��
�������ɵ���ϩ������������Ϊ2��1�������ĵ�CH4��CO2�����Ϊ_________��
����Ŀ�����к͵ζ����ⶨij�ռ���Ʒ�Ĵ��ȣ��Ը���ʵ��ش��������⣺
��1��ȷ����8��2g�������������������ʵ���Ʒ�����500mL������Һ�� ��0��100mol![]() L-1����ζ���д�����кͷ�Ӧ���Ȼ�ѧ����ʽ____________(�к�����H����57��3 kJ/mol)�������ռ���Ʒ���500mL������Һ��Ҫ�IJ���������_________________________��
L-1����ζ���д�����кͷ�Ӧ���Ȼ�ѧ����ʽ____________(�к�����H����57��3 kJ/mol)�������ռ���Ʒ���500mL������Һ��Ҫ�IJ���������_________________________��
��3���ζ������У��۾�Ӧע��_________________�����÷�̪��ָʾ���ﵽ�ζ��յ���ɫ�仯��____________________________________��
��4�������±����ݣ����㱻���ռ���Һ�����ʵ���Ũ����_____molL-1���ռ���Ʒ�Ĵ�����____������С�������λ��
�ζ����� | ������Һ �����mL�� | ������� | |
�ζ�ǰ�Ŀ̶ȣ�mL�� | �ζ���Ŀ̶ȣ�mL�� | ||
��һ�� | 10��00 | 0��40 | 20��50 |
�ڶ��� | 10��00 | 4��10 | 24��00 |
��5������ʵ�������Եζ��������ʲô�����������ƫ������ƫ����������Ӱ������
�� �۲���ʽ�ζ���Һ��ʱ����ʼ���ӣ��ζ��յ�ƽ�ӣ���ζ����____��
�� ������ƿ�ô���Һ��ϴ��Ȼ���ټ���10��00mL����Һ����ζ����______________��