��Ŀ����
����Ŀ�����ȶ���ऺϲ�����Pt2+��Cl-����ऽ���γɵIJ�����������ͬ���칹�塣��ѧ�о����������ַ��Ӷ����п������ԡ�
(1)��ष����Ǵ����ƽ�����壬��ṹ��ʽ��ͼ��ʾ![]() ����ԭ�ӵ��ӻ������ʽ��_____����ष����У���Ԫ�صĵ縺���ɴ�С��˳��Ϊ_____����ष����к���_____���� ����
����ԭ�ӵ��ӻ������ʽ��_____����ष����У���Ԫ�صĵ縺���ɴ�С��˳��Ϊ_____����ष����к���_____���� ����
(2)���ȶ���ऺϲ������д��ڵ�������������_____������ţ���
a�����Ӽ� b����λ�� c�������� d���Ǽ��Լ� e�����
(3)���ȶ���ऺϲ������У�Pt2+����λ���� 4�����������ӻ���ʽ������ sp3���������ɣ�_____________��
(4)����һ�ֶ��ȶ���ऺϲ����ӽṹ��ͼ��ʾ���÷�����_____���ӣ�ѡ���������������Ǽ���������
![]()
(5)CO(NH2)2 ������ˮ������Ҫԭ����_________________________��
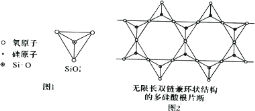
(6)Si Ԫ���� Si��O��Si �����ɿ���磬�����������壨ͼ l�����ӳ������ĵ�����˫����ͼ 2���ṹ��ͼ 2 ��ʾ�Ķ��������ӵĻ�ѧʽͨʽΪ_____���Ժ�������n �Ĵ���ʽ��ʾ����
���𰸡�sp2 N��C��H 11 bd ��Pt2+�� sp3 �ӻ����������λ������ȶ���ऺϲ�Ϊ������ṹ��������˳���칹�� �Ǽ��� CO(NH2)2 ��ˮ����֮���γ���� (Si4O11)n6n-
��������
(1)�ӽṹ��ʽ![]() ���Կ�������ԭ�ӵļ۲���Ӷ���Ϊ3���ɴ˿ɵó��ӻ������ʽ����ष����У���N��C��H����Ԫ�أ��ɷǽ����Կ�ȷ����Ԫ�صĵ縺���ɴ�С��˳����ष����У�����ԭ�Ӽ�ֻ���γ�1���� �����ɴ˿ɼ��������� ������Ŀ��
���Կ�������ԭ�ӵļ۲���Ӷ���Ϊ3���ɴ˿ɵó��ӻ������ʽ����ष����У���N��C��H����Ԫ�أ��ɷǽ����Կ�ȷ����Ԫ�صĵ縺���ɴ�С��˳����ष����У�����ԭ�Ӽ�ֻ���γ�1���� �����ɴ˿ɼ��������� ������Ŀ��
(2)���ȶ���ऺϲ������У������ܺ������Ӽ��������������������ԭ��������������λ������N5-�д��ڷǼ��Լ���
(3)���ȶ���ऺϲ������У�Pt2+����λ���� 4��������ӻ���ʽ��sp3�����ܴ���˳���칹��
(4)����һ�ֶ��ȶ���ऺϲ����ӽṹ��ͼ��ʾ���÷��ӽṹ�Գơ�
(5)CO(NH2)2���γɷ��Ӽ�����������������ˮ��
(6)ȡͼ�нṹ��Ԫ��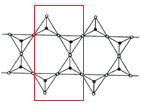 ���þ�̯���������������ӵĻ�ѧʽͨʽ��
���þ�̯���������������ӵĻ�ѧʽͨʽ��
(1)�ӽṹ��ʽ![]() ���Կ�������ԭ�ӵļ۲���Ӷ���Ϊ3����ԭ�ӵ��ӻ������ʽΪsp2����ष����У���N��C��H����Ԫ�أ��ǽ�����N>C>H�����Ԫ�صĵ縺���ɴ�С��˳��ΪN>C>H����ष����У�����ԭ�Ӽ�ֻ���γ�1���� ��������ष����к���4��̼̼�� ����2��̼���� ����5��̼���� ���������� ������ĿΪ11����Ϊ��sp2��N>C>H��11��
���Կ�������ԭ�ӵļ۲���Ӷ���Ϊ3����ԭ�ӵ��ӻ������ʽΪsp2����ष����У���N��C��H����Ԫ�أ��ǽ�����N>C>H�����Ԫ�صĵ縺���ɴ�С��˳��ΪN>C>H����ष����У�����ԭ�Ӽ�ֻ���γ�1���� ��������ष����к���4��̼̼�� ����2��̼���� ����5��̼���� ���������� ������ĿΪ11����Ϊ��sp2��N>C>H��11��
(2)���ȶ���ऺϲ������У������ܺ������Ӽ��������������������ԭ��������������λ������N5-�д��ڷǼ��Լ�����ѡbd����Ϊ��bd��
(3)���ȶ���ऺϲ������У�Pt2+����λ���� 4��������ӻ���ʽ��sp3�����ܴ���˳���칹������Ϊ��Pt2+�� sp3 �ӻ����������λ������ȶ���ऺϲ�Ϊ������ṹ��������˳���칹�塣��Ϊ����Pt2+��sp3 �ӻ����������λ������ȶ���ऺϲ�Ϊ������ṹ��������˳���칹�壻
(4)�ɶ��ȶ���ऺϲ����ӽṹ���Կ������÷��ӽṹ�Գƣ�Ϊ�Ǽ��Է��ӡ���Ϊ���Ǽ��ԣ�
(5)CO(NH2)2����Ӽ���γ����������������ˮ����ԭ��ΪCO(NH2)2 ��ˮ����֮���γ��������Ϊ��CO(NH2)2 ��ˮ����֮���γ������
(6)�����������幹�ɣ�ÿ����ԭ����4����ԭ�ӹ��������壬ȡͼ�нṹ��Ԫ�� ���ṹ��Ԫ�д��ڹ��ñ��ϵ�Oԭ��Ϊÿ���ṹ��Ԫ�ṩ1/2�����ڽṹ��Ԫ�ڵ�Oԭ����9��������4����ͶӰ��Si�غϣ��ʽṹ��Ԫ�й���Oԭ����ĿΪ9+4��
���ṹ��Ԫ�д��ڹ��ñ��ϵ�Oԭ��Ϊÿ���ṹ��Ԫ�ṩ1/2�����ڽṹ��Ԫ�ڵ�Oԭ����9��������4����ͶӰ��Si�غϣ��ʽṹ��Ԫ�й���Oԭ����ĿΪ9+4��![]() =11���ṹ��Ԫ��Siԭ����4�����ṹ��Ԫ���ϼ�Ϊ����-2����11+��+4����4=-6���ʶ��������ӵĻ�ѧʽͨʽΪ(Si4O11)n6n-����Ϊ��(Si4O11)n6n-��
=11���ṹ��Ԫ��Siԭ����4�����ṹ��Ԫ���ϼ�Ϊ����-2����11+��+4����4=-6���ʶ��������ӵĻ�ѧʽͨʽΪ(Si4O11)n6n-����Ϊ��(Si4O11)n6n-��

 �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�