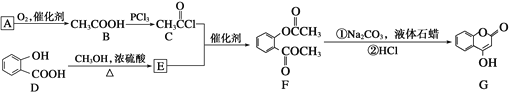
��Ŀ����
����Ŀ�����й������ʽṹ�������У�����������У� ��
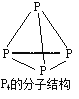
��CH3COOH������̼ԭ�ӵ��ӻ�������sp2��sp3����
��Ԫ��Geλ�����ڱ���������IVA�壬��������Ų�ʽΪ [Ar]4s24p2������P��
�۷Ǽ��Է����������и߶ȶԳ��ԣ���BF3��PCl5��H2O2��CO2�����ķ���
��Na2O��Na2O2���������Ӹ����Ȳ�ͬ
��Cu(OH)2��һ����ɫ��״�����������������ᡢҲ�����ڰ�ˮ����������������
�ް�ˮ�д�NH3��H2O��������á�����������ʾ����ϳ�NH3��H2O���ӣ����ݰ�ˮ�����ʿ�֪NH3��H2O�Ľṹʽ�ɼ�Ϊ��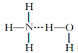
��HF����е����HCl������Ϊ HCl���ۼ�����С��HF
A. 4��B. 5��C. 6��D. 7��
���𰸡�B
��������
�ټ��У�̼ԭ�Ӽ۲���ӶԸ���=�Ҽ�����+�µ��ӶԸ���=4+0=4������Ϊsp3�ӻ���-COOH�У�̼ԭ�Ӽ۲���ӶԸ���=3+0=3���γ�3���Ҽ����¶Ե��ӣ���ȡsp2�ӻ�������ȷ��
��Geλ�ڵ������ڢ�A�壬GeΪ32��Ԫ�أ�ԭ�Ӻ�������Ų�ʽΪ[Ar]3d104s24p2������p��Ԫ�أ��ڴ���
��H2O2�ṹ���Գƣ����ڼ��Է��ӣ��۴���
��Na2Oÿ��2��Na+������1��O2-���������Ӹ�����Ϊ1��2��Na2O2ÿ��2��Na+������1��O22-���������Ӹ�����Ϊ1��2���ܴ���
��Cu(OH)2���ڼ����������ᣬ��Cu(OH)2�����ڰ�ˮ������ΪCu(OH)2�Ͱ�ˮ��Ӧ����������ӣ�������Ϸ�Ӧ��Cu(OH)2�����ԣ��ݴ���
�����Ӧ�γ���X��H-Y��ʽ�У�X��Y������N��O��FԪ��֮һ������NH3��H2O�Ľṹʽ�����ֿ��ܣ�H3N��H-O-H��H2N-H��OH2������NH3��H2O�ɵ����NH4+��OH-��ǰ�ߺ���������ȷ��
��HF��HCl���ڷ��Ӿ��壬����HF�д���H��F��H����������ã��������ʹ��HF�е����HCl���ߴ���
�ϼƹ���5�����
�ʴ�ѡB��

 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�����Ŀ������ͼ��ʾװ�ý�������ʵ�飺��������Һ������У�Ԥ���������ʵ��������ǣ� ��
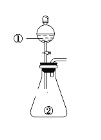
ѡ�� | �������� | �������� | Ԥ����е����� |
A | ϡ���� | ̼�������������ƵĻ����Һ | ������������ |
B | Ũ���� | ��ɰֽ��ĥ�������� | ��������ɫ���� |
C | �Ȼ�����Һ | Ũ����������Һ | ����������ɫ���� |
D | ������Һ(H2C2O4���л�ԭ��) | �������������Һ | ��Һ����ɫ |
A.AB.BC.CD.D
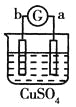
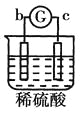
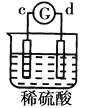
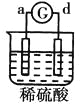
����Ŀ����a��b��c��d�ĸ������缫���йصķ�Ӧװ�ü����ַ�Ӧ�������£�
ʵ��װ�� |
|
|
|
|
����ʵ������ | a��������Сb���������� | ��Һ�е�SO42-��b���ƶ� | d���ܽ�c����������� | ������a������d�� |
�ɴ˿��ж������ֽ����Ļ��˳����
A.a>b>c>dB.b>c>d>aC.d>a>b>cD.a>b>d>c