��Ŀ����
����Ŀ��ijǿ������ɫ��Һ�п��ܺ��±������е����������ӡ�
������ | Mg2+��NH4+��Ba2+��Al3+��Fe2+ |
������ | SiO32-��MnO4-��Cl-��NO3-��SO42- |
ʵ���:ȡ������ǿ������ҺA��������ʵ�顣
ʵ���:Ϊ�˽�һ��ȷ������Һ�����,ȡ100 mLԭ��ҺA,�����Һ�еμ�1 mol��L-1��NaOH��Һ,��������������������������Һ����Ĺ�ϵ��ͼ��ʾ��
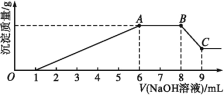
�ش���������:
(1)������ʵ��Ϳ����ƶϳ�,�ϱ��е�����һ�������ڵ���________�֡�
(2)ͨ��ʵ������ȷ������Һ��һ�����ڵ���������_________����������X�ķ�����_______________;����Z�Ļ�ѧʽΪ_____________��
(3)д��ʵ����ͼʾ��BC�ζ�Ӧ��Ӧ�����ӷ���ʽ:________________��
(4)A���Ӧ�Ĺ�������Ϊ____ g��
(5)ͨ��������Ϣ,�������Һ�������ӵ�Ũ��Ϊ________ mol��L-1��
���𰸡�4 NO3- �����Ӽ�һ��ʪ��ĺ�ɫʯ����ֽ��������X,��ֽ���� Mg(OH)2 Al(OH)3+OH-=AlO2-+2H2O 0.136 0.08
��������
ijǿ������ɫ��Һ��Fe2+��SiO32-��MnO4-��SO32-һ�������ڣ���Һ����������������ְ�ɫ����������һ������Cl-������ҺΪ�����Կ�֪һ����������ΪNO3-����Һ������������������һ������Ba2+������������������ƣ����ȣ��������������壬��һ������Mg2+��NH4+��Ϊ�˽�һ��ȷ������Һ����ɣ�ȡ100mLԭ��Һ�������Һ�еμ�1mol/L��NaOH��Һ����ͼ�в�������������������������Һ����Ĺ�ϵ��֪һ������Al3+���Դ������
(1)��ijǿ������ɫ��Һ��Fe2+��SiO32-��MnO4-��SO32-һ�������ڣ�
�ʴ�Ϊ��4��
(2)��ǿ������ɫ��Һ��Fe2+��SiO32-��MnO4-��SO32-һ�������ڣ���Һ����������������ְ�ɫ����,����һ������Cl-������������������,һ������Ba2+������������������ƣ����ȣ��������������壬��һ������Mg2+��NH4+��ͨ��ʵ��I����ȷ������Һ��һ�����ڵ���������NO3-������X�ǰ��������鷽���������Ӽ�һ��ʪ��ĺ�ɫʯ����ֽ��������x����ֽ����������Z�Ļ�ѧʽΪMg(OH)2��
�ʴ�Ϊ��NO���� �Ӽ�һ��ʪ��ĺ�ɫʯ����ֽ��������x����ֽ������Mg(OH)2��
(3)��ʵ��II��ͼ����BC�����������������������ƵĹ���, ��Ӧ�����ӷ���ʽΪ��Al(OH)3+OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al(OH)3+OH-=AlO2-+2H2O ��
(4)��BC�ζ�Ӧ�����ӷ���ʽ��Al(OH)3+OH-=AlO2-+2H2O�����ĵ�����������0.001mol�����Ժ�����������0.001mol������þ���Ӻ�������һ��������������0.005mol������þ�������ʵ���Ҳ��0.001mol��A����õ��Ĺ�����������þ0.001mol����������0.001mol��������0.001mol��58g/ mol + 0.001mol��78g/mol= 0.136g��
�ʴ�Ϊ��0.136��
(5)������Һ�д��ڵ���������NO3-������ͼ����Һ������������0.001mol������笠�������0.002mol�������ӡ�þ���Ӹ���0.001 mol�����ݵ���غ㣬������������ʵ���n= 0.001mol+0.002mol+0.002mol+0.003mol = 0.008mol��![]() ��
��
�ʴ�Ϊ��0.08��