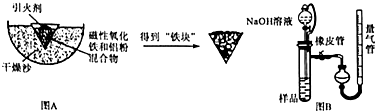
��Ŀ����
5�� �������ԭ�����ش����и�С�⣺
�������ԭ�����ش����и�С�⣺��1��25��ʱ��ijFeCl3��Һ��pH=2�������Һ����ˮ���������c��OH-��=1��10-2mol/L�������ӷ���ʽ��ʾFeCl3��Һ���ھ�ˮ��ԭ��Fe3++3H2O
 Fe��OH��3�����壩+3H+��
Fe��OH��3�����壩+3H+����2����֪Ksp��AgCl��=1.8��10-10������50mL0.018mol•L-1��AgNO3��Һ�м�50mL0.020mol•L-1�����ᣬ��Ϻ���Һ��Ag+��Ũ��Ϊ1.8��10-7��mol•L-1��
��3��NH4HSO4�ڷ����Լ���ҽҩ�����ӹ�ҵ����;�㷺������100mL 0.1mol•L-1NH4HSO4��Һ�еμ�0.1mol•L-1NaOH��Һ���õ�����ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ��ʾ���Է���ͼ��a��b��c��d��e����㣬
��ˮ�ĵ���̶�������b��
������Һ��c��OH-������ֵ��ӽ�NH3•H2O�ĵ��볣��K��ֵ����d��
����c�㣬��Һ�и�����Ũ���ɴ�С������˳����c��Na+����c��SO42-����c��NH4+����c��OH-��=c��H+����
���� ��1��ˮ�������c��OH-��=c��H+������������ˮ�����ɽ��壬������������ԣ��Ӷ���ˮ��
��2���ȼ��㷴Ӧ����Һ�������ӵ�Ũ�ȣ��ٸ���c��Ag+��=$\frac{Ksp}{c��C{l}^{-}��}$���㣻
��3����a��b��c��d��e����㣬���ݷ�Ӧ���Ĺ�ϵ��b��ǡ��������H+����Һ��ֻ�У�NH4��2SO4��Na2SO4��c��d��e������Һ������NH3•H2O����NH4��2SO4���Դٽ�ˮ�ĵ��룬��NH3•H2O����ˮ�ĵ��룮
����Һ��笠�����Ũ����һˮ�ϰ�Ũ����ӽ�ʱ����Һ��c��OH-������ֵ��ӽ�NH3•H2O�ĵ��볣��K��ֵ��
��c����Һ�����ԣ�����Һ���У�NH4��2SO4��Na2SO4��NH3•H2O���ֳɷ֣�
��� �⣺��1���Ȼ�����ǿ�������Σ���Һ�������Ӿ���ˮ������ģ�ˮ�������c��OH-��=c��H+��=10-2mol/L���Ȼ���ˮ�����������������壬ˮ�ⷽ��ʽΪFe3++3H2O?Fe��OH��3�����壩+3H+��������������ԣ�������ˮ�е�����������ܾ�ˮ��
�ʴ�Ϊ��10-2mol/L��Fe3++3H2O?Fe��OH��3�����壩+3H+��
��2��n��AgNO3��=0.05L��0.018mol/L=0.0009mol��n��HCl��=0.05L��0.020mol/L=0.001mol����������HCl������ӦAgNO3+HCl=AgCl��+HNO3�����ݷ���ʽ֪��HClʣ�࣬�����Һ��c��Cl-��=$\frac{��0.001-0.0009��mol}{0.05L+0.05L}$=10-3 mol/L����Һ��c��Ag+��=$\frac{Ksp}{c��C{l}^{-}��}$=$\frac{1.8��1{0}^{-10}}{1{0}^{-3}}$mol/L=1.8��10-7mol/L��
�ʴ�Ϊ��1.8��10-7��mol•L-1��
��3����a��b��c��d��e����㣬���ݷ�Ӧ���Ĺ�ϵ��b��ǡ��������H+����Һ��ֻ�У�NH4��2SO4��Na2SO4��c��d��e������Һ������NH3•H2O����NH4��2SO4���Դٽ�ˮ�ĵ��룬��NH3•H2O����ˮ�ĵ��룬���ˮ�ĵ���̶�������b�㣬��Һ��c��OH-������ֵ��ӽ�NH3•H2O�ĵ��볣��K��ֵ��
�ʴ�Ϊ��b��
�ڰ�ˮΪ�����Һ�ʼ��ԣ�c����Һ�����ԣ�笠�����Ũ�ȹ���e����Һ���Թ�ǿ��һˮ�ϰ�Ũ�ȹ�����Զ���d����Һ��笠�����Ũ����һˮ�ϰ�Ũ����ӽ�����Һ��c��OH-������ֵ��ӽ�NH3•H2O�ĵ��볣��K��ֵ��
�ʴ�Ϊ��d��
��c����Һ�����ԣ�����Һ���У�NH4��2SO4��Na2SO4��NH3•H2O���ֳɷ֣�b��ʱc��Na+��=c��SO42-����b��ʱc��Na+����c��SO42-��������NԪ����SԪ�صĹ�ϵ�����Եó�c��SO42-����c��NH4+������c��Na+����c��SO42-����c��NH4+����c��OH-��=c��H+����
�ʴ�Ϊ��c��Na+����c��SO42-����c��NH4+����c��OH-��=c��H+����
���� ���⿼���˽�������ʡ�����Ũ�ȴ�С�ıȽϡ��ܶȻ��������йؼ��㡢����ˮ���֪ʶ�㣬�ѶȽϴ�2�������״��⣬ע����Һ��Ϻ�������ʵ�Ũ�ȱ�Ϊԭ����һ�룬Ϊ�״��㣮

| A�� | ������ķ������Խ���ˮ����Ϊ������ˮ | |
| B�� | �þƾ���ȡ���ˮ��Һ�еĵ� | |
| C�� | �÷�Һ�ķ��������ȥ����ˮ�еĹ����������� | |
| D�� | ��ϡ�������ˣ���ȥ����ͭ���е�����п�� |
| A�� | ��ع���ʱ������Ͻ������� | |
| B�� | ��س��ʱ����������������Ӧ | |
| C�� | ��ع���ʱ��������Ӧʽ��LaNi5H6+6OH--6e-�TLaNi5+6H2O | |
| D�� | ��طŵ�ʱ������������ͨ�����·���� |
| A�� | 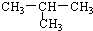 ��CH3-CH2-CH2-CH3 ��CH3-CH2-CH2-CH3 | B�� | �����C17H35COOH | ||
| C�� | �Ҷ������� | D�� | 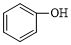 �� ��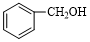 |
| A�� | 6Ħ�� | B�� | 4Ħ�� | C�� | 16Ħ�� | D�� | 8Ħ�� |
| A�� | �а�ɫ�������ɣ���Һ��ɫ��dz��ɫ����ɫ | |
| B�� | �к��ɫ�������ɣ��ϲ���Һ���ػ�ɫ | |
| C�� | ����ɫ��ζ�������������������ʹ�����ǵ�ľ����ȼ | |
| D�� | �ܿ�����Һ��ɫ���������������Ҳ������ų� |
| A�� | ���������ܶȲ���ʱ��仯 | |
| B�� | ������X��Y��Z��Ũ��֮��Ϊ1��2��2 | |
| C�� | �����ڸ����ʵ�Ũ�Ȳ���ʱ��仯 | |
| D�� | ��λʱ������0.1 mol Xͬʱ����0.2 mol Z |