��Ŀ����
����Ŀ����ѧ�仯�а�����������ת�����������о��������������к���Ҫ�����á�
�����ǻ��������ĺ��ģ���������Ļ�������������ô����ա�

��1�����dz��ô�����ѡ��Ӧ���еķ���ͼ1��ʾΪһ��������1 mol CH3OH ��O2������Ӧʱ������CO��CO2��HCHO�������仯ͼ[��Ӧ��O2(g)��������H2O(g)��ȥ]�����д��������£�CH3OH��O2��Ӧ��Ҫ����________���CO������CO2����HCHO������2HCHO(g)+O2(g)=2CO(g)+2H2O(g) ��H=________��
��������϶������������õ綯������ȼ������߽���ƶ��������������»����ʱ���綯���ṩ�ƶ���,�������͵����ģ���ɲ��������ʱ����ش��ڳ��״̬��
��2����϶���������ȼ��������Ϊȼ�ϣ����ͣ�������C8H18�ƣ���������ַ�Ӧ������1 mol ˮ��������550kJ����1 gˮ����ת��ΪҺ̬ˮ����2.5kJ��������ȼ���ȵ��Ȼ�ѧ����ʽΪ_____________��

��3����϶�����Ŀǰһ��ʹ�������أ��õ�������Ļ�����Ϊ�����������������M��ʾ��Ϊ��������Һ����ҪΪKOH��Ϊ�������Һ�������س�ŵ�ԭ��ʾ����ͼ�����ܷ�ӦʽΪ�� H2+2NiOOH![]() 2Ni(OH)2��
2Ni(OH)2��
����������Ϣ�жϣ���϶��������»����ʱ���ҵ缫��Χ��Һ��pH______�������,����С�����䡱�����õ缫�ĵ缫��ӦʽΪ_______________��
��4��Զ���ִ��ĸ��������ں�ˮ�������绯ѧ�и�ʴ�е�______��ʴ��Ϊ��ֹ���ָ�ʴ��ͨ���Ѵ�������ں�ˮ���Zn��������������Ǧ������������ֱ����Դ��______���������������������
��A��B��C����ǿ����ʣ�������ˮ�е���������������ʾ��
������ | Ag�� Na�� |
������ | NO3�� SO42 �� Cl�� |
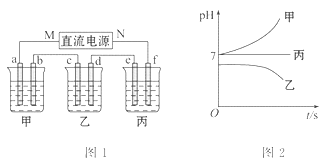
��ͼ1��ʾ��װ���У��ס��ҡ��������ձ����ηֱ�ʢ��������A��B��C������Һ���缫��Ϊʯī�缫����ͨ��Դ������һ��ʱ��������c�缫����������27�ˡ������¸��ձ�����Һ��pH����ʱ��t�Ĺ�ϵͼ��ͼ2��ʾ���ݴ˻ش��������⣺
��5��MΪ��Դ��______��(��д����������)����Ϊ__________(��д��ѧʽ)��
��6������缫f�����ɵ������ڱ�״���µ����_________��
��7��д�����ձ��еĵ��ط�Ӧ______________________��
��8�����������Һ�����Ϊ25 L�������Һ��pHΪ_______��
��9��Ҫʹ���ָ���ԭ����״̬��Ӧ����_______g______��(��д��ѧʽ)
���𰸡�HCHO -470kJ/molC8H18(l)+ ![]() O2(g)=8CO2(g)+9H2O(l) ��H=-5355kJ/mol����NiOOH+H2O+e-=Ni(OH)2+OH-��������NaCl1.4L4AgNO3��2H2O
O2(g)=8CO2(g)+9H2O(l) ��H=-5355kJ/mol����NiOOH+H2O+e-=Ni(OH)2+OH-��������NaCl1.4L4AgNO3��2H2O![]() 4Ag��O2����4HNO3122.25H2O
4Ag��O2����4HNO3122.25H2O
��������
I.��1�����Խ�ͣ���ͨ����Խ��ת��Ϊ����ӣ���ӦԽ����������ͼ������Ӧ2HCHO��g��+O2��g��=2CO��g��+2H2O��g���з�Ӧ�����������������������������÷�ӦΪ���ȷ�Ӧ�����ͼ�����ݼ��㡣
II.��2���������ʵ�״̬����Ӧ�������ı仯��ȼ���ȵĸ���Ȼ�ѧ����ʽ��д��ע��������д�Ȼ�ѧ����ʽ��
��3���������»����ʱ���綯���ṩ�ƶ��������ε�ش��ڷŵ�״̬�����ݵ���ܷ�Ӧ�͵������Һ��д�ҵ缫�ĵ缫��Ӧʽ��
��4����ˮ����ǿ���ԣ������ں�ˮ������������ʴ����ֹ������ʴ�ĵ绯ѧ�������У�������������������������ӵ�����������������
III.���ݷŵ�˳�������c�缫�����������жϵ缫���ƣ����ݵ��ʱ��ҺpH��ʱ��Ĺ�ϵȷ�����������Һ����ɣ�Ӧ�õ����غ��ԭ���غ������ؼ��㡣
I.��1������ͼ�����д��������£�CH3OH��O2��Ӧ����HCHO�Ļ��<CH3OH��O2��Ӧ����CO��CO2�Ļ�ܣ����д��������£�CH3OH��O2��Ӧ��Ҫ����HCHO������ͼʾHCHO��g����O2��Ӧ����CO��g����H2O��g���ķ�ӦΪ���ȷ�Ӧ��1molHCHO��Ӧ�ų���676-158-283��kJ=235kJ����������2molHCHO��Ӧ�ų�235kJ��2=470kJ��������Ӧ2HCHO��g��+O2��g��=2CO��g��+2H2O��g����H=-470kJ/mol��
II.��2��1molC8H18��O2��ַ�Ӧ����9molH2O��g����1molC8H18��O2��ַ�Ӧ����9molH2O��g������9��550kJ=4950kJ��9molH2O��g��ת��Ϊ9molH2O��l������9mol��18g/mol��2.5kJ/g=405kJ��1molC8H18��O2��ַ�Ӧ����9molH2O��l������4950kJ+405kJ=5355kJ������ȼ���ȵ��Ȼ�ѧ����ʽΪC8H18��l��+![]() O2��g��=8CO2��g��+9H2O��l����H=-5355kJ/mol��
O2��g��=8CO2��g��+9H2O��l����H=-5355kJ/mol��
��3���������»����ʱ���綯���ṩ�ƶ��������ε�ش��ڷŵ�״̬����ʱ��ط�ӦΪH2+2NiOOH=2Ni��OH��2���ҵ缫�ϵķ�ӦΪNiOOH��Ni��OH��2��Ni�Ļ��ϼ���+3�۽���+2�����ҵ缫�ĵ缫��ӦʽΪNiOOH+e-+H2O=Ni��OH��2+OH-����������OH-���ҵ缫��Χ��Һ��pH������
��4����ˮ����ǿ���ԣ�Զ���ִ��ĸ��������ں�ˮ������������ʴ����ֹ������ʴ�ĵ绯ѧ�������У�������������������������ӵ������������������Ѵ�������ں�ˮ���Zn������Ϊ����������������������Ϊ�˷�ֹ����ĸ�ʴ��Ҳ��������ӵ���������������������������Ǧ�����ص�ֱ����Դ�ĸ���������
III.�����ӵķŵ�˳��ΪAg+>H+>Na+����ͨ��Դ��һ��ʱ���������c�缫����������27g��c�缫������Ag������Ag�����ʵ���Ϊn��Ag��=![]() =0.25mol��c�缫�ĵ缫��ӦʽΪAg++e-=Ag�����·��ͨ���ĵ������ʵ���Ϊ0.25mol��c�缫Ϊ������d�缫Ϊ������Ag+��SO42-��Cl-���ܴ������棬����ʢ�ŵ���AgNO3��Һ�����ձ�����Һ��pH����ʱ������ƶ�������ʢ�ŵ���NaCl��Һ�����ձ�����Һ��pH����ʱ������Ʋ��䣬����ʢ�ŵ���NaNO3��Na2SO4��Һ��
=0.25mol��c�缫�ĵ缫��ӦʽΪAg++e-=Ag�����·��ͨ���ĵ������ʵ���Ϊ0.25mol��c�缫Ϊ������d�缫Ϊ������Ag+��SO42-��Cl-���ܴ������棬����ʢ�ŵ���AgNO3��Һ�����ձ�����Һ��pH����ʱ������ƶ�������ʢ�ŵ���NaCl��Һ�����ձ�����Һ��pH����ʱ������Ʋ��䣬����ʢ�ŵ���NaNO3��Na2SO4��Һ��
��5��c�缫Ϊ������c��ֱ����Դ��M������MΪ��Դ�ĸ�����NΪ��Դ�����������ձ�����Һ��pH����ʱ������ƶ���������ΪNaCl��
��6����·��ͨ���ĵ������ʵ���Ϊ0.25mol��f��N������fΪ������f�缫�ĵ缫��ӦʽΪ4OH--4e-=O2��+2H2O��f�缫�ϲ���O2���ʵ���Ϊ![]() n��e-��=0.0625mol���ڱ�״���µ����Ϊ0.0625mol��22.4L/mol=1.4L��
n��e-��=0.0625mol���ڱ�״���µ����Ϊ0.0625mol��22.4L/mol=1.4L��
��7�����ձ��е��AgNO3��Һ����Ag��O2��HNO3�������ܷ�ӦΪ4AgNO3+2H2O![]() 4Ag+O2��+4HNO3��
4Ag+O2��+4HNO3��
��8�����ձ��������缫��ӦʽΪ2H2O+2e-=H2��+2OH-����·��ͨ���ĵ������ʵ���Ϊ0.25mol����Ӧ���ɵ�OH-���ʵ���Ϊn��OH-��=n��e-��=0.25mol��c��OH-��=0.25mol��25L=0.01mol/L����c��H+��=1��10-12mol/L������Һ��pH=-lgc��H+��=12��
��9�����е���ܷ�ӦΪ2H2O![]() 2H2��+O2��~4e-����·��ͨ���ĵ������ʵ���Ϊ0.25mol��������������H2O�����ʵ���n��H2O��=
2H2��+O2��~4e-����·��ͨ���ĵ������ʵ���Ϊ0.25mol��������������H2O�����ʵ���n��H2O��=![]() n��e-��=0.125mol������ˮ������Ϊ0.125mol��18g/mol=2.25g�����б������ǵ��ˮ��Ҫʹ���ָ���ԭ��״̬Ӧ����2.25g��H2O��
n��e-��=0.125mol������ˮ������Ϊ0.125mol��18g/mol=2.25g�����б������ǵ��ˮ��Ҫʹ���ָ���ԭ��״̬Ӧ����2.25g��H2O��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�


