��Ŀ����
������ˮ��������ʱ,����˫����(H2Dz,��Ԫ����)�ѽ���������ϳɵ����Ե�����,����CCl4��ȡ�����,�Ӷ��ѽ������Ӵ�ˮ��Һ����ȫ�������������˫����(H2Dz)~CCl4������ˮ�е�Cu2+ʱ,�ȷ�����Ϸ�Ӧ:Cu2++2H2Dz Cu(HDz)2+2H+,�ټ���CCl4,Cu(HDz)2�ͺ����ױ���ȡ��CCl4�С�
Cu(HDz)2+2H+,�ټ���CCl4,Cu(HDz)2�ͺ����ױ���ȡ��CCl4�С�
(1)д��˫�����Fe3+��ϵ����ӷ���ʽ�� ,
��ȡFe3+�Ĺ�����Ҫ�������˵����,�����Һ��pH����,����������������
(2)��ͼ����˫����(H2Dz)~CCl4�����ȡijЩ�������ӵ��������,����ӳ����ȡijЩ��������ʱ���˵�pH��Χ��E%��ʾij�ֽ����������������ʽ��ȡ����İٷ��ʡ�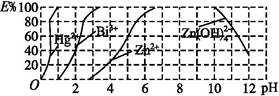
ij��ҵ��ˮ�к���Hg2+��Bi3+��Zn2+,��˫����(H2Dz)~CCl4�����ȡ��������ˮ��
������ȫ����ˮ�е�Hg2+�������,�������Һ��pH=����������
�ڵ�����pH=2ʱ,��(Bi)�Ĵ�����ʽ����������,�����ʵ���֮��Ϊ����������
����ȡ��CCl4�е�Zn(HDz)2��Һ��,��������NaOH��Һ,�����,п��ת��ˮ��Һ�С�д����Ӧ�����ӷ���ʽ:
(3)��ˮ�е��ǹ�����(H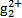 )����ת���ɹ�����(Hg2+)������˫������ϡ�ij������ˮ�к��н϶���Ȼ��ǹ�(Hg2Cl2),����������(K2S2O8)������H
)����ת���ɹ�����(Hg2+)������˫������ϡ�ij������ˮ�к��н϶���Ȼ��ǹ�(Hg2Cl2),����������(K2S2O8)������H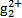 �������ṯ,д���÷�Ӧ�Ļ�ѧ����ʽ:����
�������ṯ,д���÷�Ӧ�Ļ�ѧ����ʽ:����
(1)F +3H2Dz
+3H2Dz Fe(HDz)3+3H+��Fe3+���γ�Fe(OH)3����
Fe(HDz)3+3H+��Fe3+���γ�Fe(OH)3����
(2)��1����Bi3+��Bi(HDz)3��B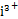 ��Bi(HDz)3=3��2
��Bi(HDz)3=3��2
��Zn(HDz)2+6OH- Zn(OH
Zn(OH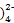 +2Dz2-+2H2O
+2Dz2-+2H2O
(3)Hg2Cl2+K2S2O8 2HgSO4+2KCl
2HgSO4+2KCl
����

 һ��һ��һ��ͨϵ�д�
һ��һ��һ��ͨϵ�д� �㽭֮��ѧҵˮƽ����ϵ�д�
�㽭֮��ѧҵˮƽ����ϵ�д� ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д�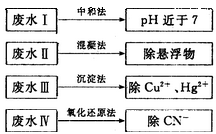
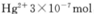 ���Ƿ�ﵽ���ŷű�_______����ǡ�����
���Ƿ�ﵽ���ŷű�_______����ǡ�����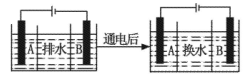
 ��
��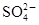 ��Ϊ�˽�һ��ȷ�ϣ�ȡ������ʵ���⣺
��Ϊ�˽�һ��ȷ�ϣ�ȡ������ʵ���⣺