��Ŀ����
��һƿ��ɫ�������Һ�����п��ܺ�Na+��Mg2+��H+��Fe3+��CO32-��Cl-��Br-�е�һ�ֻ��֣�ȡ����Һ��������ʵ�飺
����PH��ֽ���飬������Һ��ǿ����
��ȡ������Һ������������CCl4���������Ƶ���ˮ����CCl4���ԳȺ�ɫ
�۽��ڵõ�����Һ�μ���������Һ���а�ɫ�������ɣ��μ�ϡ����������ܽ⡣
����ȡ������Һ����μ���ϡNaOH��Һ��ʹ��Һ��������ת��Ϊ���ԣ��ڵμӹ����м��μ���Ϻ���Һ�о���������
��������ʵ����ʵȷ�����ش�
��1������Һ�У��϶����ڵ������� ��
��2���϶������ڵ������� ��
��3�����ܴ��ڵ������� ��
��1��H+ Br-
��2��Mg2+ Fe3+��CO32-
��3��Na+ Cl-
���������������1������Һ��ɫ��˵������Fe3+����Һ��ǿ���ԣ�˵����Һ�д���H+���������䷴Ӧ��CO32-�����ڣ��ڵ����Ƶ���ˮ����CCl4���ԳȺ�ɫ��˵����Һ�д���Br-���۲���֤��ԭ��Һ����Cl-����Ϊ�ϲ��е���Һ��ˮ�к������Ӣ���Һ�о���������˵����Һ�в���Mg2+�����Ϸ�������Һ�У��϶����ڵ�������H+ Br-
��2���϶������ڵ�������Mg2+ Fe3+��CO32-
��3�����ܴ��ڵ�������Na+ Cl-
���㣺������Һ�����Ӽ�ķ�Ӧ��������ɫ���ж�

KMnO4������Һ����ᣨH2C2O4����Һ��Ӧʱ����Һ��ɫ������ȥ��ij̽��С���òⶨ�˷�Ӧ��Һ��ɫ��ʧ����ʱ��ķ������о���������Է�Ӧ���ʵ�Ӱ�졣��ʵ����������������
������KMnO4������Һ��Ũ�ȿ�ѡ��0.02 mol��L-1��0.002 mol��L-1��
������H2C2O4��Һ��Ũ�ȿ�ѡ��0.2 mol��L-1��0.4 mol��L-1��
��ÿ��ʵ��ʱKMnO4������Һ��������Ϊ4 mL��H2C2O4��Һ��������Ϊ2mL��
��1����Ҫ̽����Ӧ��Ũ�ȡ��¶ȡ������Է�Ӧ���ʵ�Ӱ�죬ͨ���任��Щʵ��������������Ҫ���____ ��ʵ����жԱȼ��ɵó����ۡ�
��2��������������ͬ������£�ijͬѧ�ı�KMnO4������Һ��Ũ�ȣ����ʵ�����ݣ��ӻ�����ȿ�ʼ��ʱ�����±���ʾ��
| KMnO4������ҺŨ�� ��mol��L-1�� | ��Һ��ɫ����ʱ�䣨min�� | | ||
| ��һ�� | �ڶ��� | ������ | ||
| 0.02 | 14 | 13 | 11 | |
| 0.002 | 6.7 | 6.6 | 6.7 | |
����0.002 mol/L KMnO4������Һ����ʵ��ʱ��KMnO4��ƽ����Ӧ���ʣ����Ի��ǰ����Һ����仯����
�����ݱ������ݣ����ܵó�����Һ����ɫ����ʱ��Խ�̣���Ӧ����Խ�족�Ľ��ۡ�ijͬѧ������·���������ֱ�ӵó�����ɫʱ��Խ�̣���Ӧ������Խ�족���ۡ�
| KMnO4������Һ | H2C2O4��Һ | ||
| Ũ��/ mol/L | ���(ml) | Ũ��/ mol/L | �����ml�� |
| 0.02 | 2 | b | 4 |
| a | 2 | c | 4 |
�����a= ��b= ��c= ��
��3��������볣����Ka1=5.9��10-2��Ka2=6.4��10-5����KMnO4��Ӧʱ������ת��ΪCO2��H2O��
�ٲ��������Ը��������Һ��Ӧ�����ӷ���ʽΪ ��
�������£�0.1mol��L-1 KHC2O4����Һ��pH 7�������� ��
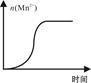
��4�����ij��ʵ�飨���£�ʱ��Һ��Mn2+���ʵ�����ʱ���ϵ��ͼ�������n(Mn2+)�ڷ�Ӧ��ʼʱ�仯����һ��ʱ�����������ԭ�� ��
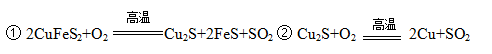
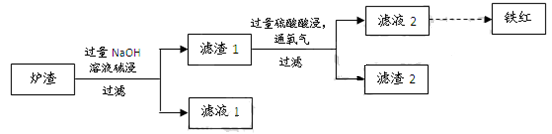
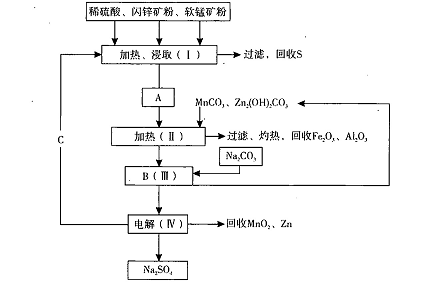
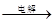 MnO2+Zn +2H2SO4��
MnO2+Zn +2H2SO4��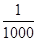 ����������CNO������
�������Σ���CNO������ Cu(HDz)2+2H+,�ټ���CCl4,Cu(HDz)2�ͺ����ױ���ȡ��CCl4�С�
Cu(HDz)2+2H+,�ټ���CCl4,Cu(HDz)2�ͺ����ױ���ȡ��CCl4�С�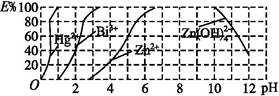
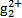 )����ת���ɹ�����(Hg2+)������˫������ϡ�ij������ˮ�к��н϶���Ȼ��ǹ�(Hg2Cl2),����������(K2S2O8)������H
)����ת���ɹ�����(Hg2+)������˫������ϡ�ij������ˮ�к��н϶���Ȼ��ǹ�(Hg2Cl2),����������(K2S2O8)������H