��Ŀ����
����Ŀ���������Ļ�����������Ҫ����;��
��1����Ԫ�������ڱ��е�λ����______�� NH3�ĵ���ʽ��______��
��2��������������̬�ĵ�ת��Ϊ����������Ĺ��̽е��Ĺ̶��������˵��һ����Ȼ���е��Ĺ̶���;��______ ���û�ѧ����ʽ��ʾ����
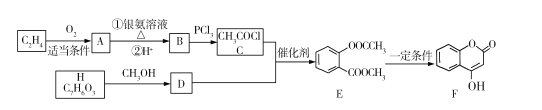
��3����ҵ�ϳɰ����˹��̵�����Ҫ������2007�껯ѧ�Ҹ�������ض�֤ʵ�������뵪���ڹ����������ϳɰ��ķ�Ӧ���̣�ʾ������ͼ:
����˵����ȷ����______��ѡ����ĸ����
a ͼ�ٱ�ʾN2��H2�����о��ǵ���
b ͼ����ͼ����Ҫ��������
c �ù��̱�ʾ�˻�ѧ�仯�а����ɻ�ѧ���Ķ��Ѻ��»�ѧ��������
��4����֪��N2��g��+3H2��g��=2NH3��g�� ��H= -92.4 kJ/mol��2H2��g��+O2��g��=2H2O��g�� ��H = -483.6kJ/mol��������ȼ����ȫȼ�����ɵ�����ˮ�������Ȼ�ѧ����ʽ��________��
��5��ˮ���£�N2H4H2O��Ϊ��ɫ������״����Һ�壬��һ����Ҫ�ľ�ϸ����ԭ�ϣ�ͨ���ڴ��������£��ô��������백��Ӧ���Ʊ����÷�Ӧ�Ļ�ѧ����ʽ��________��
���𰸡���2����VA�� ![]() N2 + O2
N2 + O2 ![]() 2NO bc 4NH3��g��+3O2��g��=2N2��g��+6H2O��g�� ��H= -1266 kJ/mol NaClO+2NH3�TN2H4H2O+NaCl
2NO bc 4NH3��g��+3O2��g��=2N2��g��+6H2O��g�� ��H= -1266 kJ/mol NaClO+2NH3�TN2H4H2O+NaCl
��������
(1)��Ԫ��λ�ڵڶ�����VA�壬NH3��N��H����ﵽ�ȶ��ṹ��
(2)���ݵ��Ĺ̶��Ķ��壬�����к��е������������ڴ���ʱ������������������Ӧ���ݴ˷�����
(3)�����ʽṹ����ѧ��Ӧ��ʵ���Ͻ��з�����
(4)���ø�˹���ɽ��з�����
(5)NaClOΪ��������NH3Ϊ��ԭ��������������������ƽ��
(1)��Ԫ��ԭ������Ϊ7��λ�ڵڶ�����VA�壬NH3�ĵ���ʽ��![]() ��
��
(2)����̬�ĵ�Ϊ�����������к��е�����������������ʱ����N2��O2![]() 2NO��
2NO��
(3)a.H2�ĽṹʽΪH��H���ǵ�����N2������Nԭ����������ʽ��ϣ���a����
b.����ͼʾ���ڡ��ۻ�ѧ���������ѣ���Ҫ������������b��ȷ��
c����ѧ��Ӧ�Ǿɼ��Ķ��Ѻ��¼����γɣ���c��ȷ��
�ʴ�ѡbc��
(4) N2��g��+3H2��g��=2NH3��g�� ��H= -92.4 kJ/mol �٣�2H2��g��+O2��g��=2H2O��g����H = -483.6kJ/mol �ڣ�NH3ȼ�յķ��̷�ʽΪ4NH3��3O2=2N2��6H2O(g)�����ݸ�˹���ɣ��ڡ�3���١�2����H=��1266kJ��mol��1���Ȼ�ѧ��Ӧ����ʽΪ4NH3(g)��3O2(g)=2N2(g)��6H2O(g) ��H=��1266kJ��mol��1��
(5)ˮ������N�ԣ�2�ۣ�NH3��N�ԣ�3�ۣ�N�Ļ��ϼ����ߣ�NH3Ϊ��ԭ����NaClOΪ�������������NaClO��NH3��N2H4��H2O��NaCl�����ݻ��ϼ�������������ƽ���ó�NaClO��2NH3=N2H4��H2O��NaCl��

����Ŀ����ͼ��Ԫ�����ڱ���һ���֣���Ҫ��ش����⣺
�� | �� | ||||||||||||||||
�� | �� | �� | �� | �� | �� | �� | �� | ||||||||||
�� | �ޡ� | �� | �� | �� | �� | �� | �� | �� | �� | �� | �� | �� | �� | �� | �ࡡ | �� | �� |
(1)Ԫ�آ������ڱ���λ����_______��Ԫ�آ۵����������Ļ�ѧʽΪ________��
(2)Ԫ�آ٢���ɻ��������ʽΪ___________��
(3)Ԫ�آڵĵ�����������������ˮ�����ȵ�Ũ��Һ��Ӧ�Ļ�ѧ����ʽΪ___��
(4)Ԫ�آ�����γɵĻ�������Ԫ�آ۵��⻯���ˮ��Һ��Ӧ�����ӷ���ʽΪ_____��
(5)Ԫ�آܢݢޢ��γɵļ����ӣ������Ӱ뾶��С�����˳��Ϊ_____(�����ӷ��ű�ʾ)��
(6)A��F������ͼ��ʾ��ת����A��B��C��DΪ��������ij��Ԫ���γɵĵ��ʣ�E��F��GΪB��C��D��A�γɵĶ�Ԫ�����G��һ�ֳ����������壬��B���Է�Ӧ����E��E��BԪ�ص���������Ϊ60%��FΪ�������ʡ�
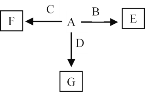
��A��F�Ļ�ѧʽ�ֱ�Ϊ_______��_______��
��B��G��Ӧ�Ļ�ѧ����ʽΪ____________________��
��C��NaOH��Һ��Ӧ�����ӷ���ʽΪ______________��