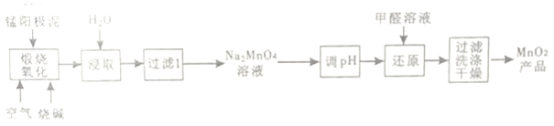
��Ŀ����
����Ŀ��(1)��(N2H4)���ӿ���ΪNH3�����е�һ����ԭ�ӱ���NH2(����)ȡ���γɵ���һ�ֵ����⻯�
���¿��������ȼ�ϣ�ȼ��ʱ�����ķ�Ӧ�ǣ�N2O4(l)��2N2H4(l)=3N2(g)��4H2O(g) ��H����1038.7 kJ��mol-1�����÷�Ӧ����4 mol N��H�����ѣ����γɵ�������________mol��
�����������ᷴӦ����N2H6SO4��N2H6SO4�����������������ͬ����N2H6SO4�����ڲ�����___�����ţ���
a�����Ӽ�����b�����ۼ�����c����λ������d�����»���
(2)��A��Ԫ������������Se���Ļ��������о�����������������Ҫ��;����ش�
��H2Se�����Ա�H2S________���ǿ������������SO32-���ӵ����幹��Ϊ________��
��H2SeO3��K1��K2�ֱ�Ϊ2.7��10-3��2.5��10-8��H2SeO4��һ��������ȫ���룬K2Ϊ1.2��10-2������ݽṹ�����ʵĹ�ϵ���ͣ�H2SeO4��H2SeO3����ǿ��ԭ��__________��
(3)[Zn(CN)4]2-��ˮ��Һ����HCHO�������·�Ӧ��4HCHO��[Zn(CN)4]2-��4H����4H2O=��Zn(H2O)4]2+��4HOCH2CN
��Zn2+��̬��������Ų�ʽΪ___________________��
����H2O���ӻ�Ϊ�ȵ������������Ϊ________��
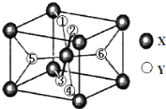
��)[Zn(CN)4]2-��Zn2+��CN����Cԭ���γ���λ���������ǿռ乹�ͣ�[Zn(CN)4]2-�Ľṹ����ʾ��ͼ��ʾΪ____________��
���𰸡�3 d ǿ ������ H2SeO3��H2SeO4�ɱ�ʾΪ(HO)2SeO��(HO)2SeO2��H2SeO3�е�SeΪ+4�ۣ���H2SeO4�е�SeΪ+6�ۣ������Ը��ߣ�����Se��O��H��O�ĵ��Ӹ���Seƫ�ƣ�Խ�����H+ 1s22s22p63s23p63d10�����Ar]3d10�� NH2- 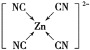
��������
(1)����Ӧ����4 mol N��H�����Ѽ�����1mol N2H4���ٸ��ݷ���ʽ���㡣
������狀������Ӽ������ۼ�����λ���������ڷ��»�����
(2)��Se��ԭ�Ӱ뾶����S��ԭ�Ӱ뾶��H2Se��Seԭ�Ӷ�Hԭ�ӵ��������������ȼ���SO32���Ӽ۲���Ӷ������ٵ����幹�ͣ���H2SeO4�ķ��ǻ�����H2SeO3�ķ��ǻ����ࡣ
(3)��ZnΪ30��Ԫ�أ������ݼ۵���N��=O������H2O���ӻ�Ϊ�ȵ����壻��[Zn(CN)4]2��Zn2+��CN����Cԭ���γ���λ����C�ṩ�¶Ե��ӡ�
(1)��N2O4(l)��2N2H4(l) = 3N2(g)��4H2O(g)�����ݷ�Ӧ����ʽ����Ӧ����4 mol N��H�����Ѽ�����1mol N2H4��������1.5mol N2��ÿ����������2������������γɵ�������3 mol���ʴ�Ϊ��3��
������狀������Ӽ������ۼ�����λ���������ڷ��»�����N2H6SO4�����������������ͬ����N2H6SO4�����ں������Ӽ������ۼ�����λ���������ڷ��»������ʴ�Ϊ��d��
(2)��Se��ԭ�Ӱ뾶����S��ԭ�Ӱ뾶��H2Se��Seԭ�Ӷ�Hԭ�ӵ�������������H2Se��ˮ�и�������������ӣ����H2Se�����Ա�H2Sǿ��SO32���Ӽ۲���Ӷ���![]() �������幹��Ϊ�����Σ��ʴ�Ϊ��ǿ�������Ρ�
�������幹��Ϊ�����Σ��ʴ�Ϊ��ǿ�������Ρ�
��H2SeO4��H2SeO3����ǿ��ԭ����H2SeO3��H2SeO4�ɱ�ʾΪ(HO)2SeO��(HO)2SeO2��H2SeO3�е�SeΪ+4�ۣ���H2SeO4�е�SeΪ+6�ۣ������Ը��ߣ�����Se��O��H��O�ĵ��Ӹ���Seƫ�ƣ�Խ�����H+���ʴ�Ϊ��H2SeO3��H2SeO4�ɱ�ʾΪ(HO)2SeO��(HO)2SeO2��H2SeO3�е�SeΪ+4�ۣ���H2SeO4�е�SeΪ+6�ۣ������Ը��ߣ�����Se��O��H��O�ĵ��Ӹ���Seƫ�ƣ�Խ�����H+��
(3)��ZnΪ30��Ԫ�أ�Zn2+��̬��������Ų�ʽΪ1s22s22p63s23p63d10���ʴ�Ϊ��1s22s22p63s23p63d10(���Ar]3d10)��
�����ݼ۵���N��=O�������H2O���ӻ�Ϊ�ȵ������������ΪNH2�����ʴ�Ϊ��NH2����
��)[Zn(CN)4]2��Zn2+��CN����Cԭ���γ���λ����C�ṩ�¶Ե��ӣ����[Zn(CN)4]2�Ľṹ����ʾ��ͼ��ʾΪ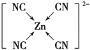 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��