��Ŀ����
12������˵����ȷ���ǣ�������| A�� | ����ʱ����ˮ�����c��H+��=1.0��10-12mol•L-1����Һ�У�K+��Na+��HCO3-��Cl-һ���ܴ������� | |
| B�� | �����£���pH=4�Ĵ�����Һϡ�ͺ���Һ���������ӵ�Ũ�Ⱦ����� | |
| C�� | �����£�pH=2�Ĵ�����Һ�������е�H+��Ϊ0.01 NA | |
| D�� | ��92 g N2O4������������У��ָ������³�ѹʱ�������������������NA |
���� A����ˮ�����c��H+��=1.0��10-12mol•L-1����Һ��Ϊ������Һ��
B�������£���pH=4�Ĵ�����Һϡ�ͺ�������Ũ�ȼ�С��Kw���䣻
C����Һ���δ֪��������n=cV���㣻
D������N2O4?2NO2��92 g N2O4��������ʵ���Ϊ1mol����Ӧ��ϵ����������ʵ�������1mol��
��� �⣺A����ˮ�����c��H+��=1.0��10-12mol•L-1����Һ��Ϊ������Һ��HCO3-���ᡢ�����Ӧ��һ�����ܹ��棬��A����
B�������£���pH=4�Ĵ�����Һϡ�ͺ�������Ũ�ȼ�С��Kw���䣬������������Ũ������B����
C����Һ���δ֪��������n=cV���㣬���ܼ���H+������C����
D������N2O4?2NO2��92 g N2O4��������ʵ���Ϊ1mol����Ӧ��ϵ����������ʵ�������1mol�������������������NA����D��ȷ��
��ѡD��
���� ���⿼����ۺϣ��漰���ӵĹ��桢pH�ļ��㡢������ʵĵ���ƽ�⼰��ѧƽ��ȣ�Ϊ��Ƶ���㣬���ճ�������֮��ķ�Ӧ����Ӧԭ��Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬�ۺ��Խ�ǿ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
3����A��B��C��D�Ŀ����Ƭ����������ʵ�飺
��A��B�õ���������ͬʱ����ϡH2SO4��Һ�У�A��Ϊ������
��C��D�õ���������ͬʱ����ϡH2SO4��Һ�У����·������D��C��
��A��C�õ���������ͬʱ����ϡH2SO4��Һ�У�C�������������ݣ�
��B��D�õ���������ͬʱ����ϡH2SO4��Һ�У�D������������Ӧ��
�ݴˣ��ж����ֽ����Ļ��˳���ǣ�������
��A��B�õ���������ͬʱ����ϡH2SO4��Һ�У�A��Ϊ������
��C��D�õ���������ͬʱ����ϡH2SO4��Һ�У����·������D��C��
��A��C�õ���������ͬʱ����ϡH2SO4��Һ�У�C�������������ݣ�
��B��D�õ���������ͬʱ����ϡH2SO4��Һ�У�D������������Ӧ��
�ݴˣ��ж����ֽ����Ļ��˳���ǣ�������
| A�� | A��C��D��B | B�� | A��B��C��D | C�� | C��A��D��B | D�� | C��B��D��A |
20��һ����̬������һ����̬ϩ�������Ƿ������̼ԭ������ͬ���� 1.0L���ֻ�������������г��ȼ�գ�����2.0LCO2��2.4Lˮ��������ͬ�����²ⶨ������������������ϩ���������Ϊ��������
| A�� | 3��1 | B�� | 1��3 | C�� | 2��3 | D�� | 3��2 |
17�� ��1��CO�Ǹ�¯��������Ҫ��Ӧ��֮һ����������Ҫ��ӦΪ��
��1��CO�Ǹ�¯��������Ҫ��Ӧ��֮һ����������Ҫ��ӦΪ��
$\frac{1}{3}$Fe2O3��s��+CO��g��?$\frac{2}{3}$Fe��s��+CO2��g������֪�÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�����£�
�÷�Ӧ��ƽ�ⳣ������ʽK=$\frac{[C{O}_{2}]}{[CO]}$����H��0�����������������=������
��2����ҵ��CO Ҳ���ںϳɼ״���
CO��g��+2H2��g��?CH3OH��g����H=-90.1kJ•mol-1��һ��ѹǿ�£����ݻ�ΪV L�������г���a mol CO��2a mol H2���ڴ��������·�Ӧ���ɼ״���ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
��p1С��p2������ڡ�����С�ڡ����ڡ�����
��100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=$��\frac{V}{a}��^{2}$��mol•L-1��-2��
�۱��������������䣬���д�ʩ���ܹ����������ϳɼ״���Ӧ�ķ�Ӧ���ʡ��������COת���ʵ���cd��������ĸ����
a��ʹ�ø�Ч���� b�����ͷ�Ӧ�¶� c��ͨ��H2d��������a mol CO��2a molH2 e�����Ͻ�CH3OH�ӷ�Ӧ������з��������
 ��1��CO�Ǹ�¯��������Ҫ��Ӧ��֮һ����������Ҫ��ӦΪ��
��1��CO�Ǹ�¯��������Ҫ��Ӧ��֮һ����������Ҫ��ӦΪ��$\frac{1}{3}$Fe2O3��s��+CO��g��?$\frac{2}{3}$Fe��s��+CO2��g������֪�÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�����£�
| �¶�/�� | 1 000 | 1 150 | 1 300 |
| ƽ�ⳣ�� | 4.0 | 3.7 | 3.5 |
��2����ҵ��CO Ҳ���ںϳɼ״���
CO��g��+2H2��g��?CH3OH��g����H=-90.1kJ•mol-1��һ��ѹǿ�£����ݻ�ΪV L�������г���a mol CO��2a mol H2���ڴ��������·�Ӧ���ɼ״���ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
��p1С��p2������ڡ�����С�ڡ����ڡ�����
��100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=$��\frac{V}{a}��^{2}$��mol•L-1��-2��
�۱��������������䣬���д�ʩ���ܹ����������ϳɼ״���Ӧ�ķ�Ӧ���ʡ��������COת���ʵ���cd��������ĸ����
a��ʹ�ø�Ч���� b�����ͷ�Ӧ�¶� c��ͨ��H2d��������a mol CO��2a molH2 e�����Ͻ�CH3OH�ӷ�Ӧ������з��������
1�����и�����ڹ����ŵ��ǣ�������
| A�� | NO${\;}_{3}^{-}$ | B�� | -Cl | C�� |  | D�� | -NO2 |
2������Ԫ��������ӵĵ����Ų�ʽ�У�ǰ��һ���ǽ���Ԫ�أ�����һ���Ƿǽ���Ԫ�ص��ǣ�������
| A�� | [Ne]3s1[Ne]3s2 | B�� | [Ar]4s1[Ne]3s23p4 | ||
| C�� | [Ne]3s2[Ar]4s2 | D�� | [He]2s22p4[Ne]3s23p5 |

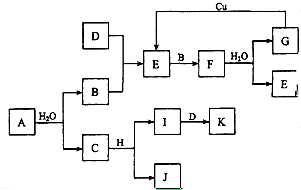 A��KΪ��ѧ��ѧ�ij������ʣ�����֮��������ͼ��ʾ��ת����ϵ����Ӧ��������ȥ������֪��ͨ��״���£�A�ǵ���ɫ���壬B��D��I������ɫ���嵥�ʣ�F�Ǻ���ɫ���壬H�ǽ������ʣ��ش��������⣺
A��KΪ��ѧ��ѧ�ij������ʣ�����֮��������ͼ��ʾ��ת����ϵ����Ӧ��������ȥ������֪��ͨ��״���£�A�ǵ���ɫ���壬B��D��I������ɫ���嵥�ʣ�F�Ǻ���ɫ���壬H�ǽ������ʣ��ش��������⣺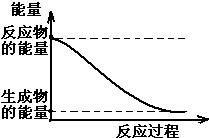 ��1��20����30�����Eyring��Pzer����ײ���۵Ļ����������ѧ��Ӧ�Ĺ���̬���ۣ���ѧ��Ӧ������ͨ������ײ������ɵģ������ڷ�Ӧ�ﵽ������Ĺ����о���һ���������Ĺ���̬����ͼ��NO2��CO��Ӧ����CO2��NO�����е������仯ʾ��ͼ��˵�������Ӧ�Ƿ��ȣ�������ȡ����ȡ�����Ӧ��NO2��CO�����������ڣ���
��1��20����30�����Eyring��Pzer����ײ���۵Ļ����������ѧ��Ӧ�Ĺ���̬���ۣ���ѧ��Ӧ������ͨ������ײ������ɵģ������ڷ�Ӧ�ﵽ������Ĺ����о���һ���������Ĺ���̬����ͼ��NO2��CO��Ӧ����CO2��NO�����е������仯ʾ��ͼ��˵�������Ӧ�Ƿ��ȣ�������ȡ����ȡ�����Ӧ��NO2��CO�����������ڣ���