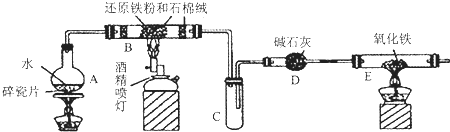
��Ŀ����
3��ij��Һ�����ֻ����NH4+��Cl-��H+��OH-�������ӣ���1��������Һ��ֻ��һ�����ʣ����������NH4Cl��д��ѧʽ����
��2��������Һ�����ԣ�����Һ��c��NH4+��=c��Cl-�������������������=��������ʱ��Һ�е�������NH4Cl��NH3•H2O��
��3��������Һ��0.02mol•L-1HCl��0.01mol•L-1NH3•H2O�������϶��ɣ�����Һ���������ӵ�Ũ���ɴ�С��˳��Ϊc��Cl-����c��H+����c��NH4+����c��OH-����
���� ��1��ij��Һ�����ֻ����NH4+��Cl-��H+��OH-�������ӣ���ֻ��һ�����ʣ�������Ϊ�Ȼ�泥�
��2�����ݵ���غ��ж���Һ��笠����Ӻ������ӵ�Ũ�ȴ�С����������غ��ж���Һ��������ɣ�
��3�����Һ������Ϊ��Ũ�ȵ��Ȼ�����Ȼ�泥���Һ�����ԣ�笠����Ӳ���ˮ�⣬��c��H+����c��NH4+����
��� �⣺��1���������Һ��ֻ����NH4+��Cl-��H+��OH-�������ӣ���ֻ��1�����ʣ�������ֻ��ΪNH4Cl��
�ʴ�Ϊ��NH4Cl��
��2������ҺΪ���ԣ���c��OH-��=c��H+�������ݵ���غ�c��Cl-��+c��OH-��=c��NH4+��+c��H+����֪��c��NH4+��=c��Cl-������Һ�л�����һˮ�ϰ����ӣ�������Ӧ��ΪNH4Cl��NH3•H2O��
�ʴ�Ϊ��=��NH4Cl��NH3•H2O��
��3��������Һ��0.02mol•L-1HCl��0.01mol•L-1NH3•H2O�������϶��ɣ���Ӧ������Ϊ��Ũ�ȵ�HCl���Ȼ�泥���������Ũ��������������������Ȼ��⡢ˮ�ĵ��뼰笠����ӵ�ˮ�⣬��c��H+����c��NH4+������Һ������Ũ�ȴ�СΪ��c��Cl-����c��H+����c��NH4+����c��OH-����
�ʴ�Ϊ��c��Cl-����c��H+����c��NH4+����c��OH-����
���� ���⿼��������Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ���ȷ����غ㡢�ε�ˮ��ԭ��Ϊ���ؼ���ע�������ж�����Ũ�ȴ�С���÷���������������ѧ���ķ������������������Ӧ��������

 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�| A�� | ��һ�����淴Ӧ�ﵽƽ��״̬ʱ�������Ӧ�������淴Ӧ������� | |
| B�� | ��ѧƽ��״̬��һ�־�ֹ״̬����Ϊ��Ӧ����������Ũ���Ѿ����ٸı� | |
| C�� | ��һ�����淴Ӧ�ﵽƽ��״̬ʱ�����������Ӧ�ڸ����������ܴﵽ������� | |
| D�� | ��ѧ��Ӧ���Ȳ�����ͨ���ı��������ı� |
| A�� | ���³�ѹ�£�8g O2����0.5NA����ԭ�� | |
| B�� | 1L 0.1mol•L-1��Na2SO4��Һ����NA��Na+ | |
| C�� | ��״���£�22.4L CCl4����NA��CCl4���� | |
| D�� | 1mol Al��ȫ��Ӧ���Al3+��ʧȥ2NA������ |
| A�� | ����Ũ�Ⱦ�Ϊ0.1 mol•L-1��HA��HB��Һ�У�����Һ��pH��СΪ��pH��HA����pH��HB�� | |
| B�� | ��0.1mol•L-1��NaA��Һ�и�����Ũ�ȹ�ϵΪ��c��Na+����c��A-����c��OH-����c��H+�� | |
| C�� | �����pH��ͬ��HA��HB��Һ���ֱ�����Ũ�ȵ�NaOH��Һ��ǡ����ȫ��Ӧ���ĵ�NaOH��Һ���HA��HB�� | |
| D�� | ����Ũ�Ⱦ�Ϊ0.1 mol•L-1��NaA��NaB��Һ�У�����Һ��pH��СΪ��pH��NaA����pH��NaB�� |
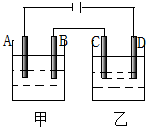 ��ͼװ�ý��е��ʵ�飨A��B��C��D��Ϊ���缫������ѡ���������Һ�����±��У�Ҫ����������ǣ���1��ͨ��һ��ʱ���׳غ��ҳص�pHֵ���½�����2���缫C��A���õ���������ʵ���֮��Ϊ2��1���ס���������е��Һ������ǣ�������
��ͼװ�ý��е��ʵ�飨A��B��C��D��Ϊ���缫������ѡ���������Һ�����±��У�Ҫ����������ǣ���1��ͨ��һ��ʱ���׳غ��ҳص�pHֵ���½�����2���缫C��A���õ���������ʵ���֮��Ϊ2��1���ס���������е��Һ������ǣ�������| ��� | �� | �� |
| 1 | NaCl | AgNO3 |
| 2 | AgNO3 | CuCl2 |
| 3 | H2SO4 | AgNO3 |
| 4 | CuSO4 | HNO3 |
| A�� | 1 | B�� | 2 | C�� | 3 | D�� | 4 |
| A�� | ����������6 mol•L-1������ | B�� | ������������ˮ | ||
| C�� | ���� | D�� | ��п�����пƬ |
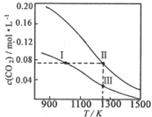 �������Ϊ1.0L���������ܱ������м�����������ͬ��̼�ۣ��ٷֱ����0.1molCO2��0.2molCO2���ڲ�ͬ�¶��·�ӦCO2��g��+C��s��?2CO��g���ﵽƽ�⣬ƽ��ʱc��CO2�����¶ȵı仯��ͼ��ʾ��ͼ�Т������������ϣ�������˵����ȷ���ǣ�������
�������Ϊ1.0L���������ܱ������м�����������ͬ��̼�ۣ��ٷֱ����0.1molCO2��0.2molCO2���ڲ�ͬ�¶��·�ӦCO2��g��+C��s��?2CO��g���ﵽƽ�⣬ƽ��ʱc��CO2�����¶ȵı仯��ͼ��ʾ��ͼ�Т������������ϣ�������˵����ȷ���ǣ�������| A�� | ״̬���״̬��CO2��ת������ͬ | |
| B�� | ��ϵ����ѹǿP����P�ܣ�״̬��2P�� ��״̬�� | |
| C�� | ��Ӧ��ƽ�ⳣ����K����K��=K�� | |
| D�� | �淴Ӧ����v�棺v�棨״̬��v�棨״̬�� |
| A | B | C | D | |
| �������� | ZnƬ��ʯī | ZnƬ��CuƬ | CuƬ��AgƬ | FeƬ��CuƬ |
| ������Һ | CuSO4��Һ | ��ˮ�ƾ� | AgNO3��Һ | ϡ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |