ЬтФПФкШн
ЁОЬтФПЁПЃЈ1ЃЉвбжЊCH3OH(l)ЕФШМЩеШШІЄHЃНЃ238.6 kJ/molЃЌCH3OH(l)ЃЋ3/2O2(g)===CO2(g)ЃЋ2H2O(g)ІЄHЃНЃa kJ/molЃЌдђ a___238.6(ЬюЁА>ЁБЁЂЁА<ЁБЛђЁАЃНЁБ)ЁЃ
ЃЈ2ЃЉЪЙCl2КЭH2O(g)ЭЈЙ§зЦШШЕФЬПВуЃЌЩњГЩHClКЭCO2ЃЌЕБга1 mol Cl2ВЮгыЗДгІЪБЪЭЗХГі145 kJШШСПЃЌаДГіИУЗДгІЕФШШЛЏбЇЗНГЬЪНЃК_________________________________ЁЃ
ЃЈ3ЃЉЗДгІmA(g)+nB(g)![]() pC(g) +qD(g)Й§ГЬжаЕФФмСПБфЛЏШчЭМЫљЪОЃЌЛиД№ЯТСаЮЪЬтЁЃ
pC(g) +qD(g)Й§ГЬжаЕФФмСПБфЛЏШчЭМЫљЪОЃЌЛиД№ЯТСаЮЪЬтЁЃ
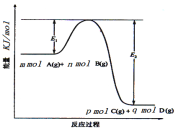
ИУЗДгІЁїH =____(гУКЌE1ЁЂE2ЪНзгБэЪО)ЃЛдкЗДгІЬхЯЕжаМгШыДпЛЏМСЃЌE1___ЃЌE2___ЃЌ(ЬюдіДѓЁЂМѕаЁЁЂВЛБф)ЁЃ
ЃЈ4ЃЉвбжЊЃКCO (g) +H2O (g)![]() H2 (g) +CO2 (g)ЦНКтГЃЪ§KЫцЮТЖШЕФБфЛЏШчЯТБэЃК
H2 (g) +CO2 (g)ЦНКтГЃЪ§KЫцЮТЖШЕФБфЛЏШчЯТБэЃК
ЮТЖШ/Ёц | 400 | 500 | 800 |
ЦНКтГЃЪ§K | 9.94 | 9 | 1 |
ЛиД№ЯТСаЮЪЬтЃК
ЂйИУЗДгІЕФІЄH=__________ЬюЁА>ЁБЁЂЁА=ЁБЛђЁА<ЁБЃЉЁЃ
ЂквбжЊдквЛЖЈЮТЖШЯТЃЌC(s) +CO2 (g)![]() 2COЃЈg)ЦНКтГЃЪ§K1ЃЛC (s) +H2O (g)
2COЃЈg)ЦНКтГЃЪ§K1ЃЛC (s) +H2O (g)![]() COЃЈg) +H2 (g)ЦНКтГЃЪ§K2ЁЃдђKЁЂK1 ЁЂK2жЎМфЕФЙиЯЕЪЧ__________________________ЁЃ
COЃЈg) +H2 (g)ЦНКтГЃЪ§K2ЁЃдђKЁЂK1 ЁЂK2жЎМфЕФЙиЯЕЪЧ__________________________ЁЃ
ЁОД№АИЁПЃМ2Cl2(g)ЃЋ2H2O(g)ЃЋC(s)===4HCl(g)ЃЋCO2(g)ЁЁІЄHЃНЃ290 kJ/mol ЁїH=-ЃЈE2-E1ЃЉkJ/molМѕаЁМѕаЁЃМK=K2/K1
ЁОНтЮіЁП
(1)ШМЩеШШЪЧ1molЮяжЪЭъШЋШМЩеЩњГЩЮШЖЈбѕЛЏЮяЗХГіЕФШШСПЃЌМзДМШМЩеЩњГЩCO2(g)КЭH2O(g)ЃЌгІИУЖјвКЬЌЫЎЮЊЮШЖЈЕФбѕЛЏЮяЃЌЗХГіЕФШШСПаЁгкШМЩеШШЃЌЙЪД№АИЮЊЃКЃМЃЛ
(2)га1mol Cl2ВЮгыЗДгІЪБЪЭЗХГі145kJШШСПЃЌ2molТШЦјЗДгІЗХШШ290kJЃЌЗДгІЕФШШЛЏбЇЗНГЬЪНЮЊЃК2Cl2(g)+2H2O(g)+C(s)ЈT4HCl(g)+CO2(g)ЁїH=-290kJmol-1ЃЌЙЪД№АИЮЊЃК2Cl2(g)+2H2O(g)+C(s)ЈT4HCl(g)+CO2(g)ЁїH=-290kJmol-1 ЃЛ
(3)гЩЭМЯёПЩжЊИУЗДгІЪЧвЛИіФмСПЩ§ИпЕФЗДгІЃЌЫљвдЪєгкЮќШШЗДгІЃЛЁїH=ЗДгІЮяЕФзмМќФм-ЩњГЩЮяЕФзмМќФмЃЌЫљвдЁїH=E1-E2ЃЛМгШыДпЛЏМСИФБфСЫЗДгІЕФЭООЖЃЌНЕЕЭЗДгІЫљашЕФЛюЛЏФмЃЌЫљвдE1КЭE2ЕФБфЛЏЪЧМѕаЁЃЌДпЛЏМСВЛИФБфЗДгІЮязмФмСПгыЩњГЩЮязмФмСПжЎВюМДЗДгІШШВЛБфЃЌЫљвдEЕФДѓаЁЖдИУЗДгІЕФЗДгІШШЮогАЯьЃЌЙЪД№АИЮЊЃКE1-E2ЃЛМѕаЁЃЛМѕаЁЃЛ
(4)ЂйИљОнЗДгІCO(g)+H2O(g)H2(g)+CO2(g)МАЛЏбЇЦНКтГЃЪ§БэДяЪНЃЌK=![]() ЃЌгЩгкЮТЖШЩ§ИпЃЌИУЗДгІЕФЛЏбЇЦНКтГЃЪ§МѕаЁЃЌЦНКтЯђзХФцЯђвЦЖЏЃЌе§ЯђЗДгІЪЧЗХШШЗДгІЃЌЫљвдЁїHЃМ0ЃЌЙЪД№АИЮЊЃКЃМЃЛ
ЃЌгЩгкЮТЖШЩ§ИпЃЌИУЗДгІЕФЛЏбЇЦНКтГЃЪ§МѕаЁЃЌЦНКтЯђзХФцЯђвЦЖЏЃЌе§ЯђЗДгІЪЧЗХШШЗДгІЃЌЫљвдЁїHЃМ0ЃЌЙЪД№АИЮЊЃКЃМЃЛ
ЂкC(s)+CO2(g)2CO(g)ЦНКтГЃЪ§K1ЃЛЂкC(s)+H2O(g)CO(g)+H2(g)ЦНКтГЃЪ§K2ЃЛЂлCO(g)+H2O(g)H2(g)+CO2(g)ЦНКтГЃЪ§KЃЛИљОнИЧЫЙЖЈТЩЃЌЂл=Ђк-ЂйЃЌЙЪK=![]() ЃЌЙЪД№АИЮЊЃКK=
ЃЌЙЪД№АИЮЊЃКK=![]() ЁЃ
ЁЃ

 ЭѕКѓалбЇАИНЬВФЭъШЋНтЖСЯЕСаД№АИ
ЭѕКѓалбЇАИНЬВФЭъШЋНтЖСЯЕСаД№АИ