��Ŀ����
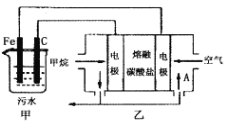
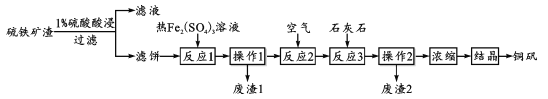
����Ŀ��ij��ҵ��ͭ���Ϻ��� Cu��CuO��CuS��CuSO4 �ȳɷ֣����øú�ͭ���Ͽ���������ͭ����[Cu(NO3)2��3H2O]�������Ĺ���������ͼ��ʾ����ش��������⣺

��1�������������������ɵķ������к�����ijɷ���______________��
��2�����ữ������Ϊ�ӿ췴Ӧ���ʣ��ɲ��õĴ�ʩ��___________________________(д��һ������)��
��3�����Լ� a ������____________���Լ�b��������______________________________________��
��4������Ӧ��һ����ʹ�� 20% HNO3 �� 10% H2O2��������������ɫ���������������Ӧ�����ӷ���ʽΪ______________________________________�����ò���ֻʹ�� 20% HNO3�����ŷ�Ӧ�Ľ��У��¶����ߣ����ִ�������ɫ���壬��ԭ���������������ʵ���֮��Ϊ_______________��
��5����ϵ�в�������Ӧ����____________��______________�����ˡ�ϴ�ӡ����
���𰸡�SO2 �����¶ȡ��ʵ����������Ũ�ȡ�������������Ĺ������ Zn��Fe��������ɣ� ��ȥ������Zn ��Fe��������һ�ն�Ӧ�� Cu+H2O2+2H+�T Cu2++2H2O 1:2 ����Ũ�� ��ȴ�ᾧ
��������
ͭ���Ϻ���Cu��CuO��CuS��CuSO4�ȳɷ֣�ͨ��������գ�2Cu+O2![]() 2CuO��2CuS+3O2
2CuO��2CuS+3O2![]() 2SO2+2CuO������Ϊ���������ټ��������ữ��ȡ���õ�����ͭ��Һ�����ȡҺ�м������ۣ����������ӷ�ӦΪCu2��+Fe=Fe2��+Cu��Ȼ����˵õ��Ĺ���Cu����ϡ������ϴͭ��ȥ���������ۣ�Ȼ�����20%�����10% H2O2��Һ��������Ӧ��Cu+H2O2+2HNO3�TCu�� NO3��2+2H2O������Һ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����õ�Cu��NO3��2��3H2O��
2SO2+2CuO������Ϊ���������ټ��������ữ��ȡ���õ�����ͭ��Һ�����ȡҺ�м������ۣ����������ӷ�ӦΪCu2��+Fe=Fe2��+Cu��Ȼ����˵õ��Ĺ���Cu����ϡ������ϴͭ��ȥ���������ۣ�Ȼ�����20%�����10% H2O2��Һ��������Ӧ��Cu+H2O2+2HNO3�TCu�� NO3��2+2H2O������Һ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����õ�Cu��NO3��2��3H2O��
��1�����գ�2Cu+O2![]() 2CuO��2CuS+3O2
2CuO��2CuS+3O2![]() 2SO2+2CuO������Ϊ�������ʴ�Ϊ��SO2��
2SO2+2CuO������Ϊ�������ʴ�Ϊ��SO2��
��2�����ữ������Ϊ�ӿ췴Ӧ���ʣ��ɲ��õĴ�ʩ�������¶ȡ��ʵ����������Ũ�ȡ�������������Ĺ�����顣
��3���Լ�a��ͭ���Ӵ���Һ���û�������ѡ�� Zn��Fe��������ɣ���
�ʴ�Ϊ�� Zn��Fe��������ɣ���
Ϊ�˳�ȥ���������ۣ���ϴҺ����Һ���ʵ���������ͬ�����Լ�bѡ��ϡ���ᣬ
�Լ�b�������dz�ȥ������Zn ��Fe��������һ�ն�Ӧ����
��4��ʹ��20%�����10% H2O2��Һ��������������ã����ӷ���ʽΪ��Cu+H2O2+2HNO3�TCu�� NO3��2+2H2O�����ò���ֻʹ��20%���ᣬ���ŷ�Ӧ�Ľ��У��¶����ߣ����ִ�������ɫ���壬���ʱ��ӦΪ������������ⷴӦ��2HNO3+H2O2=2NO2��+2H2O����ԭ��Ϊ�������⣬������Ϊ���ᣬ��ԭ���������������ʵ���֮��Ϊ 1��2��
�ʴ�Ϊ��Cu+H2O2+2H+�T Cu2++2H2O�� 1��2��
��5��������ͭ��Һ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����õ�Cu��NO3��2��3H2O
�ʴ�Ϊ�� ����Ũ�� ��ȴ�ᾧ

����Ŀ��ͭ��(��Ҫ�ɷ� CuSO4��5H2O)��һ�ֿ�����ʳƷ���ӵ�ͭǿ����������ij��������(���� CuSO4��CuSO3��Cu2O���������������Cu2S��CuS)�Ʊ�ͭ���Ĺ��չ������£�
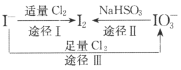
(1)��1%���������ʱ����Һ������Ϊ1:3������4��6�ν�ȡ����Ŀ����_________��
(2)���˱����к���Cu������Cu�ڡ���Ӧ1�����ܽ�����ӷ���ʽΪ________��������1����ֻ����S���ʣ���Ӧ1����Cu2S��Fe2(SO4)3��Ӧ�����ʵ���֮��Ϊ_______��
(3)����Ӧ2����ͨ�������Ŀ����_______��������ӷ���ʽ��˵������Ӧ3������ʯ��ʯ������________��
(4)Ϊ��������������������ʺͲ�Ʒ�IJ��ʣ��ڡ�Ũ����ǰ���еı�Ҫ������_____�����������ܽ����Ϣ�������˵Ľᾧ��ʽΪ_________��
t/�� | 0 | 10 | 20 | 30 | 40 | 60 | 80 |
CuSO4��5H2O/(g/100g H2O) | 23.1 | 27.5 | 32.0 | 37.8 | 44.6 | 61.8 | 83.8 |
(5)��ͭ������ʯ�ҡ�ˮ������������Ϊ1.0:0.56:100���������ͭɱ����������Һ������Ч�ɷ�ΪCuSO4��xCu(OH)2��yCa(OH)2����x=1ʱ����ȷ��y��ֵΪ____��