��Ŀ����
8��Ԫ�����ڱ���ѧϰ���ʽṹ�����ʵ���Ҫ���ߣ���ͼ��Ԫ�����ڱ���һ���֣������Ԫ�آ�-���ڱ��е�λ�ûش��������⣮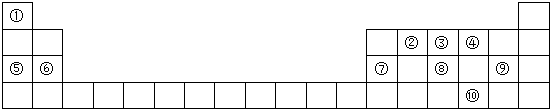
��1���ڢ�-��Ԫ���У�ԭ�Ӱ뾶��С����Cl����Ԫ�ط��ţ���
��2���۵���̬�⻯���ȶ��ԱȢܵ���̬�⻯���ȶ��������ǿ��������������ͬ������
��3���ࡢ�������������Ӧˮ���������ǿ��˳����H3PO4��HClO4�����ѧʽ��
��4���ڡ�����Ԫ���γɵ����ʵĵ���ʽ��
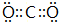 ��
����5���١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ��Ļ����д������һ�ֻ�����Ļ�ѧʽNaOH��NaClO��Na2O2�ȣ�
��6���ڢ�����У�Ԫ��ԭ��ʧ����������������Mg����Ԫ�ط��ţ������һʵ��˵��������ˮ���ҷ�Ӧ��þ��������ˮ��Ӧ��
���� ��Ԫ�������ڱ��е����λ�ÿ�֪������H������C������N������O������Na������Mg������Al������P������Cl������Se��
��1��ͬ�����������ԭ�Ӱ뾶��С��
��2���ǽ�����Խǿ����Ӧ��̬�⻯��Խ�ȶ���
��3���ǽ�����Խǿ����ۺ����������Խǿ��
��4���ڡ�����Ԫ���γɵ�����ΪCO2�ȣ�
��5��H��O��Na��Cl�е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ��Ļ�������NaOH��NaClO��Na2O2�ȣ�
��6��ͬ����������ҽ����Լ�����ʧȥ�����������������Ը��ݽ�����ˮ��Ӧ���ҳ̶ȵȽ�����֤��
��� �⣺��Ԫ�������ڱ��е����λ�ÿ�֪������H������C������N������O������Na������Mg������Al������P������Cl������Se��
��1��ͬ�����������ԭ�Ӱ뾶��С�����ڢ�-��Ԫ���У�ԭ�Ӱ뾶��С����Cl���ʴ�Ϊ��Cl��
��2���ǽ�����N��O���ǽ�����Խǿ����Ӧ��̬�⻯��Խ�ȶ�����N���⻯��������⻯���ȶ��������ʴ�Ϊ������
��3���ǽ�����P��Cl���ǽ�����Խǿ����ۺ����������Խǿ�������ԣ�H3PO4��HClO4���ʴ�Ϊ��H3PO4��HClO4��
��4���ڡ�����Ԫ���γɵ�����ΪCO2�ȣ�������̼����ʽΪ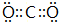 ���ʴ�Ϊ��
���ʴ�Ϊ��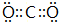 ��
��
��5��H��O��Na��Cl�е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ��Ļ�������NaOH��NaClO��Na2O2�ȣ��ʴ�Ϊ��NaOH��NaClO��Na2O2�ȣ�
��6��ͬ����������ҽ����Լ�����ʧȥ����������������Mgʧȥ��������������������ˮ���ҷ�Ӧ��þ��������ˮ��Ӧ������˵��Mgʧȥ��������������
�ʴ�Ϊ��Mg��������ˮ���ҷ�Ӧ��þ��������ˮ��Ӧ��
���� ���⿼��Ԫ�����ڱ���Ԫ�������ɣ��ѶȲ�����Ҫѧ����������Ԫ�����ڱ��ṹ��ע������ԡ��ǽ�����ǿ���Ƚ�ʵ����ʵ��

 �¿α�ͬ��ѵ��ϵ�д�
�¿α�ͬ��ѵ��ϵ�д� һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д�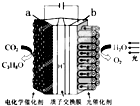 ijģ�⡰�˹���Ҷ���绯ѧʵ��װ����ͼ��ʾ����װ���ܽ�H2O��CO2ת��ΪO2��ȼ�ϣ�C3H8O��������˵����ȷ���ǣ�������
ijģ�⡰�˹���Ҷ���绯ѧʵ��װ����ͼ��ʾ����װ���ܽ�H2O��CO2ת��ΪO2��ȼ�ϣ�C3H8O��������˵����ȷ���ǣ�������| A�� | ��װ�ù���ʱ��b��������ԭ��Ӧ | |
| B�� | ÿ����1molO2����44gCO2����ԭ | |
| C�� | ��װ�ù���ʱ��H+��a����ͨ�����ӽ���Ĥ��b����Ǩ�� | |
| D�� | a�缫�ķ�ӦΪ��3CO2+18H++18e-�TC3H8O+5H2O |
| A�� | Ħ���ǹ��ʵ�λ���е�7 ������������֮һ | |
| B�� | �����Ħ��������98 g | |
| C�� | 0.5molH2 Լ����3.01��1023����ԭ�� | |
| D�� | NA������������NA ���������ӵ�������Ϊ16��1 |
| A�� | �縺�ԣ�Y��Z��X | B�� | ԭ�Ӱ뾶��X��Z��Y | ||
| C�� | ����ϼۣ�Z��X��Y | D�� | ��һ�����ܣ�Z��Y��X |
| A�� | ���ԣ�NaOH��Mg��OH��2 | B�� | ���ȶ��ԣ�HCl��HBr | ||
| C�� | ʧ����������K��Na | D�� | ԭ�Ӱ뾶��CI��S |
| A�� | 4.6% | B�� | 7.91% | C�� | 8.00% | D�� | 8.02% |
| A�� | 58.5 g�Ȼ��ƹ����к���NA���Ȼ��Ʒ��� | |
| B�� | 1mol Fe���뷴Ӧʧȥ������Ŀһ��Ϊ2NA | |
| C�� | �����ƺ�������Ӧ��ȡ�������ƣ�ÿ����1mol�������ƣ�ת�Ƶ�����Ϊ4NA | |
| D�� | �����£�46 g NO2��N2O4�Ļ�����к��еĵ�ԭ����ΪNA |
| A�� | ��״���£�22.4L CCl4����4NA�����ۼ� | |
| B�� | 1mol D2O�����ĵ�������Ϊ12NA | |
| C�� | �����£�23g NO2����NA����ԭ�� | |
| D�� | 1mol Fe2+��������H2O2��Һ��Ӧ��ת��2NA������ |