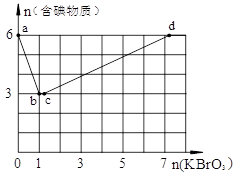
��Ŀ����
�������(K2FeO4)��һ�ּ�������������������һ������Ͷ��ˮ�������������������������£�
��ͬ���������⣺
(l)д����KOH��Һ��ͨ������Cl2������Ӧ�����ӷ���ʽ_______ ��
��2������ҺI�м���KOH�����Ŀ����______�����ţ���
| A��Ϊ��һ����Ӧ�ṩ���ԵĻ��� |
| B��ʹKClO3ת��ΪKClO |
| C������ҺI�й�����Cl2������Ӧ�����ɸ����KClO |
| D��KOH�����ܽ�ʱ��ų��϶����������������߷�Ӧ���� |
��4���������(K2FeO4)��Ϊˮ��������һ���ŵ�������ˮ��Ӧ���ɽ����������ʣ���ƽ�÷�Ӧ�����ӷ���ʽ��_______ FeO42��+_______ H2O="_______" Fe(OH)3�����壩+_______O2��+_______OH����
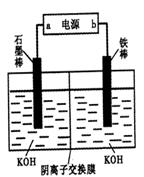
��5���ӻ��������ĽǶȿ����Ʊ�K2FeO4�Ϻõķ���Ϊ��ⷨ����װ����ͼ��ʾ���������������ĵ缫��ӦʽΪ________��

��6�����������һ�����Ͷ��ε�أ����ҺΪ����Һ���䷴ӦʽΪ��3Zn(OH)2+2Fe(OH)3+4KOH-----3Zn+2K2FeO4+8H2O,�ŵ�ʱ��صĸ�����ӦʽΪ________ ��
��1��2OH-+Cl2=ClO-+Cl-+H2O ��2�� AC ��3�� 2Fe3++3ClO-+10OH-=2FeO42-+3Cl-+5H2O 0.45
��4�� 4FeO42- +10H2O 4Fe(OH)3 (����) +3O2��+8 OH- ��5�� Fe+8OH��6e-= FeO42- -+4H2O
4Fe(OH)3 (����) +3O2��+8 OH- ��5�� Fe+8OH��6e-= FeO42- -+4H2O
��6��Zn+2OH��2e-=Zn(OH)2��Zn+2OH=Zn(OH)2+2e-��
�������������(l) KOH��Һ������Cl2������Ӧ�����ӷ���ʽΪ2OH-+Cl2=ClO-+Cl-+H2O����2������ҺI�м���KOH�����Ŀ����Ϊ��һ����Ӧ�ṩ���ԵĻ���������ҺI�й�����Cl2������Ӧ�����ɸ����KClO����ѡ��ΪA. C����3�� ��Ӧ���з��������ӷ�Ӧ����ʽΪ2Fe3++3ClO-+10OH-=2FeO42-+ Cl-+5H2O .�ڷ���ʽ��ÿ����2mol��FeO42-ת�Ƶ���6mol.����������3mol.����n(K2FeO4)= 59.4g��198g/mol=0.3mol�������������������ʵ���Ϊ3��2��0.3mol=0.45mol.��4�� �������(K2FeO4)ˮ������ӷ���ʽΪ4FeO42- +10H2O  4Fe(OH)3 (����) +3O2��+8 OH-��5�� ��ⷨ���Ʊ�K2FeO4�Ϻõķ�����Fe���������缫��ӦΪFe+8OH��6e-= FeO42- +4H2O��ʯī���������缫��ӦΪ��O2+ 2H2O+4e-=4OH��.��6��������طŵ�ʱ��صĸ�����ӦʽΪZn+2OH��2e-=Zn(OH)2��
4Fe(OH)3 (����) +3O2��+8 OH-��5�� ��ⷨ���Ʊ�K2FeO4�Ϻõķ�����Fe���������缫��ӦΪFe+8OH��6e-= FeO42- +4H2O��ʯī���������缫��ӦΪ��O2+ 2H2O+4e-=4OH��.��6��������طŵ�ʱ��صĸ�����ӦʽΪZn+2OH��2e-=Zn(OH)2��
���㣺�������Ͷ��ˮ�������������(K2FeO4)���Ʒ���ԭ��������ת�Ƽ�������صȷ�Ӧԭ����֪ʶ��

 ��ҵ����ϵ�д�
��ҵ����ϵ�д� ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д� �����ܾ�ϵ�д�
�����ܾ�ϵ�д�Fe2����I�������ֳ����Ļ�ԭ�����ӡ�
��1����FeSO4��Һ�еμ���ˮ����Һ��dz��ɫ��ɻ�ɫ����Ӧ�����ӷ���ʽΪ________________________����KI��Һ�еμ���ˮ����Һ����ɫ��ɻ�ɫ����Ӧ�����ӷ���ʽΪ______________________��
��2������FeSO4��Һ��KI��Һ����ˮ��2% KSCNΪ�Լ�֤��I���Ļ�ԭ��ǿ��Fe2�������ʵ�鷽�����������ʵ�鲽�衢Ԥ������ͽ��ۡ�
| ʵ�鲽�� | Ԥ����������� |
| ����1��ȡ2mLFeSO4��Һ��2mLKI��Һ������Թ��У��ٵμ�1~2����ˮ�� | ������Һ��ɻ�ɫ�� ���ۣ� �� |
| ����2��__________________________ __________________________________ | ���� �� ���ۣ� |
��3������ ���ṩ���Լ�֤���������Ļ����������ԣ�2�ۣ�ʵ������������ǣ�ȡ������Ʒ����ˮ�� ��
��4����2mol FeI2��Һ��ͨ��2.5mol Cl2ʱ����д���ܵ����ӷ���ʽ�� ��
 Fe(OH)3��5OH��]
Fe(OH)3��5OH��]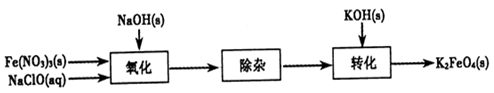

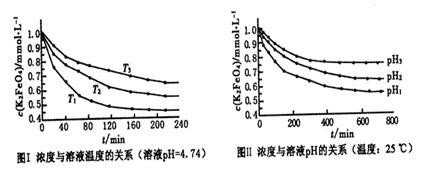
 ClO2��+___________________��û����ƽ��
ClO2��+___________________��û����ƽ��