��Ŀ����
17��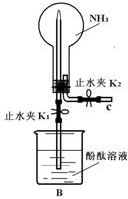 ��1����ͬѧ����ͼBװ����NH3��Ȫʵ�飬�ر�K2����K1����������Բ����ƿ��һ��ʱ�����ƿ���к�ɫ��Ȫ����
��1����ͬѧ����ͼBװ����NH3��Ȫʵ�飬�ر�K2����K1����������Բ����ƿ��һ��ʱ�����ƿ���к�ɫ��Ȫ������������γ���Ȫ��ԭ����������ײ����B��ˮ�Ӵ����ܽ⣬�Ӷ�����ѹǿƽ�⣻
���÷���ʽ��ʾ��̪����ԭ��NH3+H2O�TNH3��H2O�TNH4++OH-��
��2����ͬѧ��Bװ����NH3��Cl2��Ӧ��ʵ�飮
����1���ر�K1����K2ͨ��Cl2����ƿ�г��ְ��̣�д����Ӧ�Ļ�ѧ����ʽ��8NH3+3Cl2=6NH4Cl+N2��
����2��ͨ��Cl2��ǡ����ȫ��Ӧ�ر�K2����K1����ƿ�е������ǣ��γ���Ȫ��ʵ����Ϻ���ƿ����Һ�����ռ��ƿ�ݻ��ķ����ǣ�$\frac{7}{8}$��
���� ��1���ٹر�K2����K1����������Բ����ƿ������������ײ����B��ˮ�Ӵ����ܽ⣬�Ӷ�����ѹǿƽ�⣻
�ڰ�����ˮ��Ӧ����һˮ�ϰ���һˮ�ϰ���������������ӣ��Լ��ԣ�
��2������1������Ϊ�Ȼ�泥��������백����Ӧ�����Ȼ�狀͵�����
����2��ͨ��Cl2��ǡ����ȫ��Ӧ�ر�K2����K1����ƿ��ѹǿ��С��ˮ������ֻ�е���������ˮ��
��� �⣺��1���ٹر�K2����K1����������Բ����ƿ������������ײ����B��ˮ�Ӵ����ܽ⣬�Ӷ�����ѹǿƽ�⣬��ѹ������ƿ��ѹ�����γ���Ȫ��
�ʴ�Ϊ������������ײ����B��ˮ�Ӵ����ܽ⣬�Ӷ�����ѹǿƽ�⣻
�ڰ�������ˮ������ˮ��Ӧ����һˮ�ϰ����ٵ�������笠����Ӻ����������ӣ���Һ�ʼ��ԣ���ӦΪNH3+H2O�TNH3��H2O�TNH4++OH-��
�ʴ�Ϊ��NH3+H2O�TNH3��H2O�TNH4++OH-��
��2������1������Ϊ�Ȼ�泥��������백����Ӧ�����Ȼ�狀͵�������ӦΪ8NH3+3Cl2=6NH4Cl+N2���ʴ�Ϊ��8NH3+3Cl2=6NH4Cl+N2��
����2��ͨ��Cl2��ǡ����ȫ��Ӧ�ر�K2����K1����ƿ��ѹǿ��С��ˮ�������۲쵽�γ���Ȫ��ֻ�е���������ˮ��������ƿ�ݻ�Ϊ8V����8NH3+3Cl2=6NH4Cl+N2��֪����������������ΪV������ʵ����Ϻ���ƿ����Һ�����ռ��ƿ�ݻ��ķ�����$\frac{8V-V}{8V}$=$\frac{7}{8}$���ʴ�Ϊ���γ���Ȫ��$\frac{7}{8}$��
���� ���⿼��ʵ��װ���ۺ�Ӧ�ü���Ȫʵ�飬Ϊ��Ƶ���㣬������Ȫʵ���ԭ�������ʵ�����Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬��Ŀ�ѶȲ���

| A�� | 8 | B�� | 18 | C�� | 10 | D�� | 28 |
| A�� | ������������ˮ���ʻ��о����������ζ | |
| B�� | ����ͼ״���һ��������Ҳ�ܷ���������Ӧ | |
| C�� | ������ӦҲ����ȡ����Ӧ | |
| D�� | ������Ӧ����Ҫϡ���������� |
| A�� | 94g | B�� | 14g | C�� | 47g | D�� | 7g |
| A�� | CH3OH | B�� | CH3CH2CH3 | ||
| C�� | 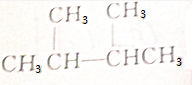 | D�� | CH3COOCH2CH3 |
| A�� | NaOH | B�� | KMnO4 | C�� | KSCN | D�� | ���� |
 CH3COOCH3+H2O��
CH3COOCH3+H2O��