��Ŀ����
17���Ȼ����dz�����ˮ��������ij�Ȼ�����FeCl3•6H2O����Ʒ��������FeCl2���ʣ���Ҫ�ⶨ����FeCl3•6H2O������������ʵ�鰴���²�����У�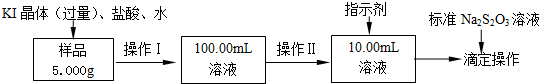
��֪�й����ӷ���ʽΪ��2Fe3++2I-�T2Fe2++I2��I2+2S2O32-�T2I-+S4O62-
��1��ȡ�����Ȼ�����Ʒ����50mL��ˮ�У�����Ƭ��Һ����ֺ��ɫ����Ӧ�����ӷ���ʽΪ��Fe3++3H2O?Fe��OH��3+3H+��
��2�����������õ��IJ����������ձ����������⣬��������100mL����ƿ����ͷ�ιܣ����������ƣ���
��3������������õ���������d��ѡ���ţ���
a��50mL�ձ� b��10mL��Ͳ �� c��20mL��Ͳ d��25mL�ζ���
��4��ָʾ���ǵ�����Һ����ﵽ�ζ��յ�����������һ�α�Һ����ʱ����ƿ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ��
��5���ζ�ʱ������Ũ��Ϊ0.1000mol/L�ı�Na2S2O3��Һ18.17mL������Ʒ��FeCl3•6H2O����������Ϊ98.3%��
��6��Ҫ����Ʒ�Ȼ����е�����FeCl2���ʳ�ȥ�����õ��Լ���bd��ѡ���ţ���
a������ b����ˮ������c����ˮ d��˫��ˮ��
���� ij�Ȼ�����FeCl3•6H2O����Ʒ��������FeCl2���ʣ���Ҫ�ⶨ����FeCl3•6H2O������������ȡ��Ʒ5.000g����KI���塢�����ˮ�ܽ⣬ȷ����100.00ml��Һ��ȷ��ȡ10.00ml��Һ����ƿ�м������ָʾ��������������Ʊ���Һ�ζ����յ㣬��ȡ���ı���Һ���������Ϸ�Ӧԭ���ͷ�Ӧ��Ӧ��ϵ�õ���Ԫ�ص����ʵ���������õ�FeCl3•6H2O������������
��1���Ȼ���������Һ�����ˮ�м������������������壻
��2����������һ�����ʵ���Ũ�ȵ���Һ����Ҫ�������У���Ͳ����ͷ�ιܡ��ձ�����������һ����������ƿ��
��3��������������Һ����ľ�ȷ�ȿ�֪��100.00mL����Һ��Ҫ����������ȡ��
��4���������۱���ɫ�����Na2S2O3��Һ����͵ⵥ�ʷ�Ӧ����Һ��ɫ��Ϊ��ɫ�Ұ���Ӳ���ɫ��
��5�����ݷ�Ӧ�Ķ�����ϵ����õ���ע����Һ����ı仯��
��6��Ҫ����Ʒ�Ȼ����е�����FeCl2���ʳ�ȥ����Ҫ����������������������Ϊ�����ӣ�����������������������µ����ʣ�
��� �⣺��1��ȡ�����Ȼ�����Ʒ����50mL��ˮ�У�����Ƭ�̣�Һ����ֺ��ɫ�����ɵ��������������壬��Ӧ�����ӷ���ʽΪFe3++3H2O?Fe��OH��3+3H+��
�ʴ�Ϊ��Fe3++3H2O?Fe��OH��3+3H+��
��2��������һ�����ʵ���Ũ�ȵ���Һ����Ҫ�������У���Ͳ����ͷ�ιܡ��ձ�����������һ����������ƿ��
�ʴ�Ϊ��100mL����ƿ����ͷ�ιܣ�
��3��100.00mL����Һ��Ҫ����������ȡ���ձ��Ǵ�����ȡ����Ͳֻ�ܾ�ȷ��0.1mL�������õζ��ܾ�ȷ��0.01mL��ѡ�õζ�����ȡ��Һ10.00mL����Һ��
�ʴ�Ϊ��d��
��4���������۱���ɫ�����Na2S2O3��Һ����͵ⵥ�ʷ�Ӧ�����һ�α�Һ����ʱ����Һ��ɫ�仯Ϊ��ɫ�Ұ���Ӳ���ɫ��˵���ﵽ��Ӧ�յ㣬
�ʴ�Ϊ�����һ�α�Һ����ʱ����ƿ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ��
��5��2Fe3++2I-��2Fe2++I2��I2+2S2O32-��2I-+S4O62-��2FeCl3-6H2O��2Fe3+��I2��2S2O32-���ζ�ʱ��10.00ml��Һ�еⵥ������Ũ��Ϊ0.1000mol/L�ı�Na2S2O3��Һ18.17mL��FeCl3-6H2O�����ʵ���=0.1000mol/L��0.01817L=0.001817mol������Ʒ��100.00mL��Һ������FeCl3•6H2O�����ʵ���Ϊ0.01817mol����������=$\frac{0.01817mol��270��g/mol}{5.0g}$��100%=98.3%��
�ʴ�Ϊ��98.3%��
��6��Ҫ����Ʒ�Ȼ����е�����FeCl2���ʳ�ȥ����Ҫ����������������������Ϊ�����ӣ�����������������������µ����ʣ�
a�����ۺ������ӷ�Ӧ�����ܺ� �������ӷ�Ӧ����a�����ϣ�
b����ˮ����������������Ϊ�����ӣ��Ҳ������µ����ʣ���b���ϣ�
c����ˮ�������������ӣ��������������ӣ���c�����ϣ�
d��˫��ˮ����������������Ϊ�����ӣ��������ⱻ��ԭΪˮ�����������ʣ���d���ϣ�
�ʴ�Ϊ��bd��
���� ���⿼����������ɺ����ʵ�ʵ����֤��ʵ��̽�������������仯�������ʵķ���Ӧ�ã����ʳ��ӣ��ζ�ʵ��ⶨ���ʺ����ļ���Ӧ���жϣ���Ŀ�Ѷ��еȣ�

 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�| A�� | MgSO4�TMg2++SO42- | B�� | Fe��OH��3�TFe3++3OH- | ||
| C�� | NaHCO3�TNa++HCO3- | D�� | KAl��SO4��2�TK++Al3++2SO42- |
| A�� | �п��Ľ���Na��¶�ڿ����У������ı����䰵�������ķ�ӦΪ2Na+O2�TNa2O2 | |
| B�� | 4.6gNa��O2��ȫ��Ӧ������7g����ʱʧȥ���ӵ����ʵ���Ϊ0.2 mol | |
| C�� | Na��ϡ���ᷴӦ�����ӷ���ʽΪ2Na+2H+�T2Na++H2�� | |
| D�� | ������NaͶ�뵽CuSO4��Һ�У����г���������������ų� |
| A�� | ��Ҫ���Ȳ��ܷ����ķ�Ӧ���������ȷ�ӦҲ�����Ƿ��ȷ�Ӧ | |
| B�� | 1 molǿ����1molǿ����кͷ�Ӧ���ų������������к��� | |
| C�� | ���淴Ӧ2SO2+O2?2SO3��ƽ��״̬�£����ֺ��º����������м���һ������O2��Qc��С��K���䣬O2ת���ʼ�С | |
| D�� | �����������ɷ���״����������ߴ�Ч�� |
| A�� | Na2CO3��Һ��c ��Na+����c ��CO32-��֮�� | |
| B�� | 0.2 mol•L-1��CH3COOH��Һ��0.1 mol•L-1��������c ��H+��֮�� | |
| C�� | pH=7�İ�ˮ�루NH4��2SO4�Ļ����Һ�У�c ��NH4+����c ��SO42-��֮�� | |
| D�� | pH=12��Ba��OH��2��Һ��pH=12��KOH��Һ�����ʵ����ʵ���Ũ��֮�� |
| A�� | ˮ | B�� | ����ͭ��Һ | C�� | ϡ���� | D�� | NaCl��Һ |
| A�� | �о綾 | B�� | �и�ʴ�� | C�� | ��֧��ȼ�� | D�� | ���ܹ������� |
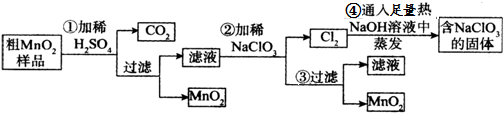
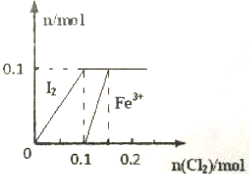 ijʵ��С����100mL FeI2��Һ����ͨ��Cl2�������η������·�Ӧ��
ijʵ��С����100mL FeI2��Һ����ͨ��Cl2�������η������·�Ӧ��