��Ŀ����
16����Ԫ�صĻ�����Ӧ��ʮ�ֹ㷺����ش���1�����ȼ��Һ̬ƫ�����£�C2H8N2������Һ̬N2O4�������������߷�Ӧ�ų��������ȣ�������������Ⱦ�������ˮ����֪�����£�1gȼ����ȫȼ���ͷų�������Ϊ42.5kJ����÷�Ӧ���Ȼ�ѧ����ʽΪC2H8N2��l��+2N2O4��l��=2CO2��g��+3N2��g��+4H2O��l����H=-2550 kJ•mol-1��

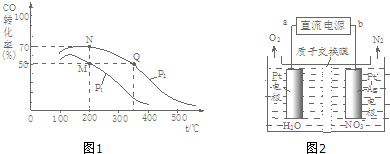
��2��298Kʱ����2L�̶�������ܱ������У��������淴Ӧ��2NO2��g��?N2O4��g����H=-a kJ/mol��a��0����N2O4�����ʵ���Ũ����ʱ��仯��ͼ1����ƽ��ʱ��N2O4��Ũ��ΪNO2��2�����ش��������⣺
��298kʱ���÷�Ӧ��ƽ�ⳣ��Ϊ6.67L•mol-1����ȷ��0.01����
������������Ǵ���ƽ��״̬����A��
A�����������ܶȱ��ֲ��䣻 B������������ɫ���ٱ仯�� C����ѹ�㶨ʱ
������Ӧ��398K���У�ijʱ�̲��n��NO2��=0.6moln��N2O4��=1.2mol�����ʱV��������V���棩�����������������=������
��3��NH4HSO4�ڷ����Լ���ҽҩ�����ӹ�ҵ����;�㷺������100mL 0.1mol•L-1NH4HSO4��Һ�еμ�0.1mol•L-1NaOH��Һ���õ�����ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ2��ʾ���Է���ͼ��a��b��c��d��e����㣮
��b��ʱ����Һ�з���ˮ�ⷴӦ��������NH4+��
����c�㣬��Һ�и�����Ũ���ɴ�С������˳��c��Na+����c��SO42-����c��NH4+����c��OH-��=c��H+����
��d��e���Ӧ��Һ�У�ˮ����̶ȴ�С��ϵ��d��e�����������������=������
���� ��1�������ƫ�����µ����ʵ������ٸ������ʵ���֮�ȵ��������������Ӧ�ȣ�Ȼ��д���Ȼ�ѧ����ʽ��
��2������ͼ��֪N2O4��ƽ��Ũ��Ϊ0.6mol/L���ﵽƽ��ʱ��N2O4��Ũ��ΪNO2��2������NO2��ƽ��Ũ��Ϊ0.3mol/L������ƽ�ⳣ������ʽ���㣻
��A��������������䣬�ݻ��㶨�����������ܶ�Ϊһ��ֵ��
B������������ɫ���䣬˵��NO2��Ũ�ȱ��ֺ㶨��
C����Ӧ2NO2��g��?N2O4��g����ѹ�㶨ʱ�������淴Ӧ������ȣ�
�۷�ӦΪ���ȷ�Ӧ�������¶ȣ�Kֵ��С�������֪��ʱ��Ũ����Q=K��298K����K��398K������Ӧ���淴Ӧ�����ƶ������V��������V���棩��
��3����a��b��c��d��e����㣬���ݷ�Ӧ���Ĺ�ϵ��b��ǡ��������H+����Һ��ֻ�У�NH4��2SO4��Na2SO4��
��c����Һ�����ԣ�����Һ���У�NH4��2SO4��Na2SO4��NH3•H2O���ֳɷ֣�
�۸��ݼ�����Һ�У�����������Ũ��Խ��ˮ�ĵ���̶�ԽС���з�����
��� �⣺��1��1gȼ����ȫȼ���ͷų�������Ϊ42.5kJ����1molƫ��������ȫȼ���ͷų�������Ϊ42.5kJ��60=2550kJ�����Ȼ�ѧ����ʽΪ��C2H8N2��l��+2N2O4��l���T2CO2��g��+3N2��g��+4H2O��l����H=-2550 kJ/mol��
�ʴ�Ϊ��C2H8N2��l��+2N2O4��l��=2CO2��g��+3N2��g��+4H2O��l����H=-2550 kJ•mol-1��
��2������ͼ��֪N2O4��ƽ��Ũ��Ϊ0.6mol/L���ﵽƽ��ʱ��N2O4��Ũ��ΪNO2��2������NO2��ƽ��Ũ��Ϊ0.3mol/L����K=$\frac{c��{N}_{2}{O}_{4}��}{{c}^{2}��N{O}_{2}��}$=$\frac{0.6}{��{0.3��}^{2}}$=6.67��
�ʴ�Ϊ��6.67��
��A����Ӧ���������ȫ����̬���ʣ�������������䣬�ݻ�Ϊ2L���ֺ㶨���ɦ�=$\frac{m}{V}$��֪������ܶ�Ϊһ��ֵ�����������ܶȱ��ֲ��䲻һ������ƽ��״̬����A��ȷ��
B������������ɫ����˵��NO2��Ũ�Ȳ��䣬˵����Ӧ���ڻ�ѧƽ��״̬����B����
C����Ӧ2NO2��g��?N2O4��g����һ�����������С�ķ�Ӧ����ѹ�㶨ʱ�������淴Ӧ������ȣ�˵����Ӧ���ڻ�ѧƽ��״̬����C����
�ʴ�Ϊ��A��
�۷�ӦΪ���ȷ�Ӧ�������¶ȣ�Kֵ��С���ܱ����������Ϊ2L����˵�N2O4��Ũ��Ϊ0.6mol/L��NO2��Ũ��Ϊ0.3mol/L��Ũ����Q=$\frac{c��{N}_{2}{O}_{4}��}{{c}^{2}��N{O}_{2}��}$=$\frac{0.6}{��{0.3��}^{2}}$=6.67=K��298K����K��398K������Ӧ���淴Ӧ�����ƶ����ʣ�V��������V���棩���ʴ�Ϊ������
��3����a��b��c��d��e����㣬���ݷ�Ӧ���Ĺ�ϵ��b��ǡ��������H+����Һ��ֻ�У�NH4��2SO4��Na2SO4����Һ�з���ˮ�ⷴӦ��������NH4+���ʴ�Ϊ��NH4+��
��c����Һ�����ԣ�����Һ���У�NH4��2SO4��Na2SO4��NH3•H2O���ֳɷ֣�b��ʱc��Na+��=c��SO42-����c��ʱc��Na+����c��SO42-��������NԪ����SԪ�صĹ�ϵ�����Եó�c��SO42-����c��NH4+������Һ������Ũ�ȴ�СΪ��c��Na+����c��SO42-����c��NH4+����c��OH-��=c��H+����
�ʴ�Ϊ��c��Na+����c��SO42-����c��NH4+����c��OH-��=c��H+����
��d��e��Һ��Ϊ������Һ����Һ�е�����������������ˮ�ĵ��룬������������Һ���Խ��ˮ�ĵ������ԽС����d��ˮ�ĵ���̶ȴ���e��ˮ�ĵ��룬
�ʴ�Ϊ������
���� ���⿼���Ȼ�ѧ����ʽ����д��ƽ�ⳣ���ļ��㡢ƽ����ƶ�������Ũ�ȴ�С�Ƚϡ�����ˮ���֪ʶ�㣬��Ŀ�Ѷ��еȣ�����֪ʶ��϶࣬�ۺ��Խ�ǿ����ֿ�����ѧ���ķ�������������������Ũ�ȴ�С�Ƚϳ�������ˮ�⡢������ʵĵ������Ͽ��飬ȷ������Ũ�ȴ�СʱҪ��ϵ���غ㡢�����غ����������

| A�� | CCl4 | B�� | CH3COOH | C�� | CH3CH2OH | D�� | CaCO3 |
 �о������ḻ��CO2��ȫ������Ϊ��̼Դ�������ǰӦ����㷺��̼Դ��ʯ�ͺ���Ȼ��������������Ҷ���ݽߵ�Σ����ͬʱ�ֿɻ�����CO2�ۻ�������������ЧӦ��ʵ��CO2������ѭ����
�о������ḻ��CO2��ȫ������Ϊ��̼Դ�������ǰӦ����㷺��̼Դ��ʯ�ͺ���Ȼ��������������Ҷ���ݽߵ�Σ����ͬʱ�ֿɻ�����CO2�ۻ�������������ЧӦ��ʵ��CO2������ѭ������1��Ŀǰ��ҵ����һ�ַ�������CO2��H2��230�����������ת�����ɼ״�������ˮ��������ͼ��ʾ��ѹ������0.5molCO2��1.5molH2ת���ʴ�80%ʱ�������仯ʾ��ͼ�����жϸ÷�Ӧ�ﵽ��ѧƽ��״̬��������bd��������ĸ��
a��������ѹǿ���䡡���� b��H2������������䡡����
c��c��H2��=3c��CH3OH������d���������ܶȲ���
e��2��C=O���ѵ�ͬʱ��6��H-H���ѣ�
��2������ͬ����CO��g����H2O�ֱ�ͨ�뵽���Ϊ2L�ĺ����ܱ������У����з�Ӧ��CO��g��+H2O��g��?CO2��g��+H2��g�����õ������������ݣ�
| ʵ���� | �¶� �� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min[ | ||
| CO | H2O | H2 | CO | |||
| 1 | 650 | 4 | 2 | 1.6 | 2.4 | 6 |
| 2 | 900 | 2 | 1 | 0.4 | 1.6 | 3 |
| 3 | 900 | a | b | c | d | t |
��ʵ��3�У���ƽ��ʱ��CO��ת���ʴ���ˮ��������a/b��ֵ0��$\frac{a}{b}$��1�������ֵ��ȡֵ��Χ����
��ʵ��4����900��ʱ���ڴ������м���CO��H2O��CO2��H2��Ϊ1 molʱ�����ʱv��������v���棩���������������=������
��3����֪�ڳ��³�ѹ�£�
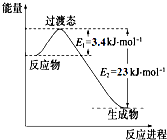
��2CH3OH��l��+3O2��g��=2CO2��g��+4H2O��g����H=-1275.6kJ/mol
��2CO��g��+O2��g��=2CO2��g����H=-566.0kJ/mol
��H2O��g��=H2O��l����H=-44.0kJ/mol
д���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��CH3OH��l��+O2��g���TCO��g��+2H2O��l����H=-442.8kJ•mol-1��
��4����֪������һ�ֶ�Ԫ���ᣬ����������Һ�����ԣ������£���10mL��0.01mol/L H2C2O4��Һ�еμ�10mL0.01mol/L��NaOH��Һ���Ƚ���Һ�и�������Ũ�ȵĴ�С��ϵc��Na+����c��HC2O4-����c��H+����c��C2O42-����c��OH-����
��5���Լ��ѡ�����������������ҺΪԭ�ϣ�ʯīΪ�缫�ɹ���ȼ�ϵ�أ��õ�صĸ�����ӦʽΪCH3OCH3-12e-+16OH-=2CO32-+11H2O��
| A�� | �Ȼ�����Һ����ˮ | B�� | NaOH��Һ����ˮ | ||
| C�� | ���������Һ����ˮ | D�� | NaHCO3��Һ����ˮ |
| A�� | CO2��SiO2 | B�� | Na2O2��H2O2 | C�� | NaCl��HCl | D�� | CCl4��CS2 |
�� ���� | ��A | ��A | ��A | IVA | VA | VIA | VIIA | O�� |
| 1 | ||||||||
| 2 | G | H | D | |||||
| 3 | B | C | E | |||||
| 4 | F | A | ||||||
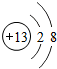 ��GԪ�ص���������ﻯѧʽCO2��H���⻯��Ļ�ѧʽNH3
��GԪ�ص���������ﻯѧʽCO2��H���⻯��Ļ�ѧʽNH3��2��HԪ�صĵ��ʵĵ���ʽ
 ��
����3��A-H����Ԫ����
��a��ԭ�Ӱ뾶����Ԫ����K
��b�����ʵĻ�ԭ����ǿ��Ԫ����K
��c������������Ӧˮ����������ǿ����HClO4
��d������������Ӧˮ���������ǿ����KOH��
��4������ʽ��ʾAE2���γɹ���

��5��B��C������������Ӧˮ���ﷴӦ�����ӷ���ʽAl��OH��3+OH-�TAlO2-+2H2O��
| A�� | ��ϩ�����ʽC2H4 | B�� | ��ͪ�ķ���ʽC3H6O | ||
| C�� | ���Ȼ�̼�ĵ���ʽΪ��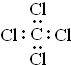 | D�� | �Ҵ��Ľṹ��ʽC2H6O |