��Ŀ����
7��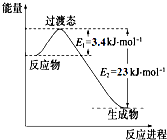 �о������ḻ��CO2��ȫ������Ϊ��̼Դ�������ǰӦ����㷺��̼Դ��ʯ�ͺ���Ȼ��������������Ҷ���ݽߵ�Σ����ͬʱ�ֿɻ�����CO2�ۻ�������������ЧӦ��ʵ��CO2������ѭ����
�о������ḻ��CO2��ȫ������Ϊ��̼Դ�������ǰӦ����㷺��̼Դ��ʯ�ͺ���Ȼ��������������Ҷ���ݽߵ�Σ����ͬʱ�ֿɻ�����CO2�ۻ�������������ЧӦ��ʵ��CO2������ѭ������1��Ŀǰ��ҵ����һ�ַ�������CO2��H2��230�����������ת�����ɼ״�������ˮ��������ͼ��ʾ��ѹ������0.5molCO2��1.5molH2ת���ʴ�80%ʱ�������仯ʾ��ͼ�����жϸ÷�Ӧ�ﵽ��ѧƽ��״̬��������bd��������ĸ��
a��������ѹǿ���䡡���� b��H2������������䡡����
c��c��H2��=3c��CH3OH������d���������ܶȲ���
e��2��C=O���ѵ�ͬʱ��6��H-H���ѣ�
��2������ͬ����CO��g����H2O�ֱ�ͨ�뵽���Ϊ2L�ĺ����ܱ������У����з�Ӧ��CO��g��+H2O��g��?CO2��g��+H2��g�����õ������������ݣ�
| ʵ���� | �¶� �� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min[ | ||
| CO | H2O | H2 | CO | |||
| 1 | 650 | 4 | 2 | 1.6 | 2.4 | 6 |
| 2 | 900 | 2 | 1 | 0.4 | 1.6 | 3 |
| 3 | 900 | a | b | c | d | t |
��ʵ��3�У���ƽ��ʱ��CO��ת���ʴ���ˮ��������a/b��ֵ0��$\frac{a}{b}$��1�������ֵ��ȡֵ��Χ����
��ʵ��4����900��ʱ���ڴ������м���CO��H2O��CO2��H2��Ϊ1 molʱ�����ʱv��������v���棩���������������=������
��3����֪�ڳ��³�ѹ�£�
��2CH3OH��l��+3O2��g��=2CO2��g��+4H2O��g����H=-1275.6kJ/mol
��2CO��g��+O2��g��=2CO2��g����H=-566.0kJ/mol
��H2O��g��=H2O��l����H=-44.0kJ/mol
д���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��CH3OH��l��+O2��g���TCO��g��+2H2O��l����H=-442.8kJ•mol-1��
��4����֪������һ�ֶ�Ԫ���ᣬ����������Һ�����ԣ������£���10mL��0.01mol/L H2C2O4��Һ�еμ�10mL0.01mol/L��NaOH��Һ���Ƚ���Һ�и�������Ũ�ȵĴ�С��ϵc��Na+����c��HC2O4-����c��H+����c��C2O42-����c��OH-����
��5���Լ��ѡ�����������������ҺΪԭ�ϣ�ʯīΪ�缫�ɹ���ȼ�ϵ�أ��õ�صĸ�����ӦʽΪCH3OCH3-12e-+16OH-=2CO32-+11H2O��
���� ��1��������Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g�������淴Ӧ����ƽ��ʱ��ͬһ���ʱ�ʾ�����淴Ӧ������ȣ�����ֵ�Ũ�ȡ��������䣬�ɴ�����������һЩ�����䣬�ж�ƽ��״̬��������Ӧ�淴Ӧ���з����仯�����������ɱ仯�����仯��˵������ƽ�⣻
��2������������ʽ����ƽ��ʱ��������ʵ��������ڷ�Ӧǰ�������������䣬�����ʵ�������Ũ�ȴ���ƽ�ⳣ������ʽK=$\frac{c��C{O}_{2}����c��{H}_{2}��}{c��CO����c��{H}_{2}O��}$���㣻
�ڸ��ݷ���ʽ��֪���μӷ�ӦCO��ˮ�����ʵ�����ȣ����CO��ת���ʴ���ˮ������ת�����жϣ�
�����ڷ�Ӧǰ�������������䣬�����ʵ�������Ũ�ȼ��������Ũ����Qc����ƽ�ⳣ����ȣ��жϷ�Ӧ���з������ж�v��������v���棩����Դ�С��
��3�����ݸ�˹���ɣ���֪�Ȼ�ѧ����ʽ�����ʵ���ϵ�����мӼ�����Ŀ���Ȼ�ѧ����ʽ����Ӧ��Ҳ������Ӧ�ļ��㣻
��4��������������Ƶ����ʵ�����ȣ�����ǡ�÷�Ӧ����NaHC2O4������������Һ�����ԣ�˵��HC2O4-�ĵ���̶ȴ�����ˮ��̶ȣ���������������ˮ�ĵ����HC2O4-�ĵ��룬��c��H+����c��C2O42-������Һ������������Ũ����С��
��5��ԭ��ظ�������������Ӧ�������ڸ���ʧȥ���ӣ���������������̼�����ˮ��
��� �⣺��1��������Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����
a����Ӧ�ں��º�ѹ�£�������ѹǿʼ�ղ��䣬��a������
b����Ӧ��H2������������䣬��Ӧ����ƽ�⣬��b��ȷ����
c������û��ָ�������淴Ӧ���ʣ�c��H2��=3c��CH3OH������˵����Ӧ����ƽ�⣬��c������
d����Ӧ�ں��º�ѹ�£��淴Ӧ���л�����������ʵ�����С�������ݻ���С������������������䣬��������ܶ������������ܶȲ���ʱ��˵����Ӧ����ƽ�⣬��d��ȷ��
e��2��C=O���ѵ�ͬʱ��6��H-H���ѣ�����ʾ����Ӧ���ʣ���Ӧʼ�հ��ñ������У�����˵������ƽ�⣬��e����
��ѡ��bd��
��2����CO��g��+H2O��g��?CO2��g��+H2��g����
��ʼ��mol����2 1 0 0
ת����mol����0.4 0.4 0.4 0.4
ƽ�⣨mol����1.6 0.6 0.4 0.4
���ڷ�Ӧǰ�������������䣬�����ʵ�������Ũ�ȼ���ƽ�ⳣ������ƽ�ⳣ��K=$\frac{c��C{O}_{2}����c��{H}_{2}��}{c��CO����c��{H}_{2}O��}$=$\frac{0.4��0.4}{1.6��0.6}$=$\frac{1}{6}$��
�ʴ�Ϊ��$\frac{1}{6}$��
�ڸ��ݷ���ʽ��֪���μӷ�ӦCO��ˮ�����ʵ�����ȣ��������ʵ�����Ϊx��CO��ת���ʴ���ˮ������ת���ʣ���$\frac{x}{a}$��$\frac{x}{b}$����a��b����Ϊ0��������0��$\frac{a}{b}$��1��
�ʴ�Ϊ��0��$\frac{a}{b}$��1��
����900��ʱ���ڴ������м���CO��H2O��CO2��H2��Ϊ1 molʱ����ʱŨ����Qc=$\frac{1��1}{1��1}$=1��K=$\frac{1}{6}$���ʷ�Ӧ���淴Ӧ���У����ʱv��������v���棩��
�ʴ�Ϊ������
��3����֪����2CH3OH��l��+3O2��g��=2CO2��g��+4H2O��g����H=-1275.6kJ/mol
��2CO��g��+O2��g��=2CO2��g����H=-566.0kJ/mol
��H2O��g��=H2O��l����H=-44.0kJ/mol
���ݸ�˹���ɣ�$\frac{1}{2}$[��-��]+2���۵ã�CH3OH��l��+O2��g���TCO��g��+2H2O��l�������H=$\frac{1}{2}$��[��-1275.6kJ•mol-1��-��-566.0kJ•mol-1��]+2����-44.0kJ•mol-1��=-442.8kJ•mol-1��
���Ȼ�ѧ����ʽΪ��CH3OH��l��+O2��g���TCO��g��+2H2O��l����H=-442.8kJ•mol-1��
�ʴ�Ϊ��CH3OH��l��+O2��g���TCO��g��+2H2O��l����H=-442.8kJ•mol-1��
��4����10mL 0.01mol•L-1��H2C2O4��Һ�μ�10mL 0.01mol•L-1NaOH��Һ����������������ǡ�÷�Ӧ����NaHC2O4�����ڲ���������Һ��ʾ���ԣ���HC2O4-�ĵ���̶ȴ�����ˮ��̶ȣ�����c��C2O42-����c��H2C2O4������������������ˮ�ĵ����HC2O4-�ĵ��룬��c��H+����c��C2O42-����HC2O4-��ˮ��̶Ƚ�С����c��HC2O4-����c��C2O42-�������������£�ˮ�ĵ���̶�ԶԶС��HC2O4-�ĵ��룬����Һ������������Ũ����С��������Һ�и�����Ũ�ȴ�СΪ��c��Na+����c��HC2O4-����c��H+����c��C2O42-����c��OH-����
�ʴ�Ϊ��c��Na+����c��HC2O4-����c��H+����c��C2O42-����c��OH-����
��5��ԭ��ظ�������������Ӧ�������ڸ���ʧȥ���ӣ���������������̼�����ˮ���ʸ����缫��ӦʽΪ��CH3OCH3-12e-+16OH-=2CO32-+11H2O��
�ʴ�Ϊ��CH3OCH3-12e-+16OH-=2CO32-+11H2O��
���� ��������ƴ������Ŀ���漰��ѧƽ��״̬�жϡ���ѧƽ����㡢ƽ�ⳣ�����㼰Ӧ�á��Ȼ�ѧ����ʽ��д������Ũ�ȱȽϡ�ԭ��ص缫��Ӧʽ��д�ȣ���ѧƽ�ⳣ���ļ��㼰Ӧ���ǽ�����߿�����֪ʶ�㣬��ѧ���ۺ������Ŀ��飬�Ѷ��еȣ�

| A�� | ��ϩ�ͼ�����һ�������¶�������������Ӧ | |
| B�� | ��ϩ�;���ϩ����ʹ������Ȼ�̼��Һ��ɫ | |
| C�� | ��ϩ�ͱ�����ʹ���Ը��������Һ��ɫ | |
| D�� | ��ϩ�����鶼���������������ӳɷ�Ӧ |
ʵ��һ ���Ʋ��궨������Һ��Ũ��ȡ����������250mL 0.2mol•L-1�Ĵ�����Һ����0.2mol•L-1�Ĵ�����Һϡ�ͳ�����Ũ�ȵ���Һ������NaOH����Һ�����������Һ��Ũ�Ƚ��б궨��
�ش��������⣺
��1������250mL 0.2mol•L-1������Һʱ��Ҫ�õ��IJ�����������Ͳ���ձ�������������ͷ�ιܺ�250mL����ƿ��
��2��Ϊ�궨������Һ��ȷŨ�ȣ���0.2000mol��L-1��NaOH��Һ��20.00mL������Һ���еζ������εζ�����NaOH��Һ��������£�
| ʵ����� | 1 | 2 | 3 | 4 |
| ����NaOH��Һ�������mL�� | 20.05 | 20.00 | 18.80 | 19.95 |
ʵ��� ̽��Ũ�ȶԴ������̶ȵ�Ӱ��
��pH�Ʋⶨ25��ʱ��ͬŨ�ȵĴ����pH��������£�
| ����Ũ�ȣ�mol•L-1�� | 0.0010 | 0.0100 | 0.0200 | 0.1000 | 0.2000 |
| pH | 3.88 | 3.38 | 3.23 | 2.88 | 2.73 |
��1�����ݱ������ݣ����Եó�������������ʵĽ��ۣ�����Ϊ�ó��˽��۵������ǣ�0.0100mol•L-1�����pH����2�����ϡ��10��ʱ��pH�ı仯ֵС��1��
��2���ӱ��е����ݣ������Եó���һ���ۣ����Ŵ���Ũ�ȵļ�С������ĵ���̶Ƚ����������С�����䡱��

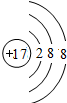
 ��1124Na��
��1124Na��
 ��
��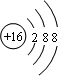 ��
��