题目内容
1. 请你利用所学反应原理知识解决下列问题:
请你利用所学反应原理知识解决下列问题:(1)若已知两个反应:
①C(s)+2H2(g)=CH4(g)△H1=a kJ•mol-1;
②C(s)+1212O2(g)=CO(g)△H2=b kJ•mol-1;
则2CH4(g)+O2(g)=2CO(g)+4H2(g)△H=2(b-a)kJ•mmol-1(用含a、b的式子表示);
(2)碱性镁锰干电池是新开发的一种干电池,比普通锌锰干电池具有更加优越的性能,具有较大应用前景,其工作时总反应为:Mg+2MnO2+H2O=Mg(OH)2+Mn2O3;则工作时,正极发生还原反应(填反应类型),写出负极的电极反应式:Mg+2OH--2e-=Mg(OH)2;
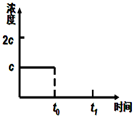
(3)在一定温度下1L的密闭容器放入足量草酸钙(固体所占体积忽略不计)发生反应:CaC2O4(s)═CaO(s)+CO(g)+CO2(g),若前5min 内生成CaO的质量为11.2g,则该段时间内v(CO)=0.04mol•L-1•min-1;若某时刻达到平衡时c(CO2)=c;t0时刻,将容器体积缩小为原来的一半并固定不变,在t1时刻再次达到平衡,请在图中画出t0以后此体系中CO2的浓度随时间变化的图象;
(4)某温度下数据:草酸(H2C2O4)的K1=5.4×10-2,K2=5.4×10-5;醋酸的K=1.75×10-5;碳酸的 K1=4.2×10-7,K2=4.5×10-11;Ksp(CaC2O4)=5.0×10-9;Ksp(CaCO3)=2.5×10-9
①用醋酸溶液鉴别CaC2O4和CaCO3两种白色固体的实验现象是一种固体溶解同时产生气泡逸出,另一种固体无现象;
②向0.6mol/L的Na2CO3溶液中加入足量 CaC2O4粉末后(忽略溶液体积变化),充分搅拌,发生反应:CO32-(aq)+CaC2O4(s)?CaCO3(s)+C2O42-(aq),静置后沉淀转化达到平衡,求此时溶液中的c(C2O42-)(不考虑其他诸如水解之类副反应,写出计算过程).
分析 (1)由盖斯定律可知,②×2-①×2得2CH4(g)+O2(g)═2CO(g)+4H2(g)以此计算△H;
(2)正极是MnO2得到电子发生还原反应生成Mn2O3,负极镁失电子生成的镁离子和氢氧根结合生成氢氧化镁沉淀;
(3)前5min 内生成CaO的质量为11.2g,n(CO)=n(CaO)=11.2g56g/mol11.2g56g/mol=0.2mol,则v(CO)=0.2mol1L•5min0.2mol1L∙5min=0.04mol•L-1•min-1;温度不变,平衡常数不变,t0时刻,将容器体积缩小为原来的一半并固定不变,c(CO2)=2c,在t1时刻再次达到平衡时,c(CO2)=c,作图即可;
(4)①醋酸的酸性大于碳酸的酸性,小于草酸的酸性,故醋酸与CaC2O4不反应,与CaCO3反应有气泡逸出;
②该反应的K=c(C2O42−)c(CO32−)c(C2O42−)c(CO32−)=c(C2O42−)c(Ca2+)c(CO32−)c(Ca2+)c(C2O42−)c(Ca2+)c(CO32−)c(Ca2+)=Ksp(CaC2O4)Ksp(CaCO3)Ksp(CaC2O4)Ksp(CaCO3)=5.0×10−92.5×10−95.0×10−92.5×10−9=2.0,设c(CO32-)转化了x,则生成c(C2O42-)=x,剩余c(CO32-)=(0.6-x),结合K的表达式计算.
解答 解:(1)①C(s)+2H2(g)═CH4(g)△H1=a kJ•mol-1;
②C(s)+1212O2(g)═CO(g)△H2=b kJ•mol-1;
依据盖斯定律:②×2-①×2得2CH4(g)+O2(g)═2CO(g)+4H2(g)△H=2(b-a)kJ•mmol-1;
故答案为:2(b-a)kJ•mmol-1;
(2)正极是MnO2得到电子发生还原反应生成Mn2O3,负极镁失电子生成的镁离子和氢氧根结合生成氢氧化镁沉淀,Mg-2e-+2OH-=Mg(OH)2,
故答案为:还原;Mg+2OH--2e-=Mg(OH)2;
(3)前5min 内生成CaO的质量为11.2g,n(CO)=n(CaO)=11.2g56g/mol11.2g56g/mol=0.2mol,则v(CO)=0.2mol1L•5min0.2mol1L∙5min=0.04mol•L-1•min-1;温度不变,平衡常数不变,t0时刻,将容器体积缩小为原来的一半并固定不变,c(CO2)=2c,在t1时刻再次达到平衡时,c(CO2)=c,作图如下: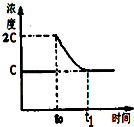 ,
,
故答案为:0.04mol•L-1•min-1; ;
;
(4)①醋酸的酸性大于碳酸的酸性,小于草酸的酸性,故醋酸与CaC2O4不反应,与CaCO3反应有气泡逸出;
故答案为:一种固体溶解同时产生气泡逸出,另一种固体无现象;
②该反应的K=c(C2O42−)c(CO32−)c(C2O42−)c(CO32−)=c(C2O42−)c(Ca2+)c(CO32−)c(Ca2+)c(C2O42−)c(Ca2+)c(CO32−)c(Ca2+)=Ksp(CaC2O4)Ksp(CaCO3)Ksp(CaC2O4)Ksp(CaCO3)=5.0×10−92.5×10−95.0×10−92.5×10−9=2.0,
设c(CO32-)转化了x,则生成c(C2O42-)=x,剩余c(CO32-)=(0.6-x),
可得方程:x(0.6−x)x(0.6−x)=2.0,解得x=0.4 mol•L?1;
答:此时溶液中的c(C2O42-)为0.4 mol•L?1.
点评 本题考查盖斯定律、原电池原理的应用、弱酸的性质等知识,综合性较强,难度中等.

 天天向上一本好卷系列答案
天天向上一本好卷系列答案 小学生10分钟应用题系列答案
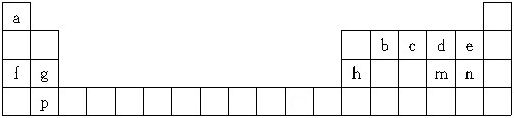
小学生10分钟应用题系列答案| 元素 | 相关信息 |
| A | 原子核外L层电子数是K层的2倍 |
| B | 其一种单质被称为地球生物的“保护伞” |
| C | 焰色反应呈黄色 |
| D | 与B同主族的短周期元素 |
| E | 可形成多种氧化物,其中一种为具有磁性的黑色晶体 |
(1)E在元素周期表中的位置第四周期第Ⅷ族;D原子结构示意图是

(2)B、C、D的简单离子半径由大到小的顺序为(用化学符号表示,下同)S2->O2->Na+,B、D的简单气态氢化物中稳定性较大的是H2O
(3)B、C的单质按物质的量比1:2形成的化合物中化学键的类型为离子键、好几件;该化合物电子式为
 .
.(4)E的一种具有磁性的黑色晶体发生铝热反应的化学方程式是3Fe3O4+8Al高温_高温–––––9Fe+4Al2O3.
| 选项 | 叙述I | 叙述II |
| A | Mg有还原性 | 电解MgCl2饱和溶液可制备Mg |
| B | AgCl难溶于酸 | 用盐酸和AgNO3溶液检验Cl- |
| C | Ba(OH)2易溶于水 | 可配制1.0mol?L-1的Ba(OH)2溶液 |
| D | NH4Cl为强酸弱碱盐 | 用加热法除去NaCl中的NH4Cl |
| A. | A | B. | B | C. | C | D. | D |
| A. | K+、Al3+、CO32-、Cl- | B. | Na+、H+、SO42-、SiO32- | ||
| C. | H+、NH4+、SO42-、I- | D. | H+、Fe2+、Cl-、ClO- |
| 初始浓度/(mol•L-1) | 初始速率/mol•L-1s-1 | |
| c(NO) | c(O2) | |
| 0.010 | 0.010 | 2.5×10-3 |
| 0.010 | 0.020 | 5.0×10-1 |
| 0.030 | 0.020 | 4.5×10-2 |
| 容器编号 | 温度(℃) | 起始物质的量(mol) | 平衡物质的量(mol) | |
| CH3OH(g) | CH3OCH3(g) | H2O(g) | ||
| Ⅰ | 387 | 0.20 | 0.080 | 0.080 |
| Ⅱ | 387 | |||
| Ⅲ | 207 | 0.20 | 0.090 | 0.090 |
(1)反应的△H小于0(填“大于”或“小于”)判断的理由是反应Ⅰ、Ⅲ起始量相同,Ⅰ的CH3OCH3(g)含量低,说明降温正向移动,正反应为放热过程△H<0,容器Ⅰ到达平衡所需的时间为20min反应速率v(CH2OH)为0.004mol•L-1•min-1,列式求算387℃时该反应的平衡常数K1=4
(2)容器Ⅱ达平衡时,压强是容器Ⅰ的两倍,CH3OH的体积分数和容器Ⅰ中的相同,CH3OH起始的物质的量为0.40mol;平衡时CH3OCH3(g)的体积分数为0.4
(3)t分钟后容器Ⅲ达到平衡,t大于20min(填“大于”、“等于”或“小于”)判断的理由是温度越低,反应速率越小,达到平衡时所需的时间越长请在图中分别画出容器Ⅰ和容器Ⅱ中CH3OH(g)浓度变化的曲线示意图

(4)208℃,若向3L容器中充入0.9molCH3OH(g),0.6molCH3OCH3(g)和0.3molH2O(g),则起始时该反应的v正>v逆(填“>”“<”或“=”)
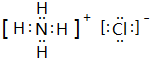 .
.