��Ŀ����
11��һ���¶��£����������ԼΪ2.0L�ĺ����ܱ������з�����Ӧ��2CH3OH��g��=CH3OCH3��g��+H2O��g��| ������� | �¶ȣ��棩 | ��ʼ���ʵ�����mol�� | ƽ�����ʵ�����mol�� | |
| CH3OH��g�� | CH3OCH3��g�� | H2O��g�� | ||
| �� | 387 | 0.20 | 0.080 | 0.080 |
| �� | 387 | |||
| �� | 207 | 0.20 | 0.090 | 0.090 |
��1����Ӧ�ġ�HС��0������ڡ���С�ڡ����жϵ������Ƿ�Ӧ����ʼ����ͬ�����CH3OCH3��g�������ͣ�˵�����������ƶ�������ӦΪ���ȹ��̡�H��0��������ƽ�������ʱ��Ϊ20min��Ӧ����v��CH2OH��Ϊ0.004mol•L-1•min-1����ʽ����387��ʱ�÷�Ӧ��ƽ�ⳣ��K1=4
��2���������ƽ��ʱ��ѹǿ���������������CH3OH������������������е���ͬ��CH3OH��ʼ�����ʵ���Ϊ0.40mol��ƽ��ʱCH3OCH3��g�����������Ϊ0.4
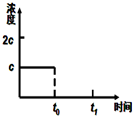
��3��t���Ӻ�������ﵽƽ�⣬t����20min������ڡ��������ڡ���С�ڡ����жϵ��������¶�Խ�ͣ���Ӧ����ԽС���ﵽƽ��ʱ�����ʱ��Խ������ͼ�зֱ����������������CH3OH��g��Ũ�ȱ仯������ʾ��ͼ

��4��208�棬����3L�����г���0.9molCH3OH��g����0.6molCH3OCH3��g����0.3molH2O��g��������ʼʱ�÷�Ӧ��v����v�����������������=����
���� ��1�����ݢ�͢�ó����¶�Խ��ƽ��ʱ����Խ�࣬��˵������ƽ��������Ӧ�����ƶ����ݴ��ж��ʱ䣻��������ʽ���V=$\frac{��c}{��t}$����������CH2OH�ķ�Ӧ���ʣ�K=$\frac{c��CH{\;}_{3}OCH{\;}_{3}��•c��H{\;}_{2}O��}{c{\;}^{2}��CH{\;}_{3}OH��}$������ƽ�ⳣ����
��2���������ƽ��ʱ��ѹǿ���������������CH3OH������������������е���ͬ����Ϊ��Чƽ�⣬�ָ÷�ӦΪ�����������ķ�Ӧ������ֻҪ��Ӧ��ɱ�������Ч����Чƽ������ʵ����������ͬ���ݴ˷�����
��3���¶�Խ�ͣ���Ӧ����ԽС���ﵽƽ��ʱ�����ʱ��Խ�������������������������ݣ���ʼʱCH3OH��g��Ũ����ͬ��ƽ��ʱ������CH3OH��g��Ũ��Ϊ$\frac{0.20-0.08��2}{2}$=0.02mol/L��ƽ��ʱ������CH3OH��g��Ũ��Ϊ$\frac{0.20-0.09��2}{2}$=0.01mol/L��
��4�����ݻ�ѧƽ�ⳣ����Ũ���̵���Դ�С�жϷ�Ӧ�������Ũ����С��ƽ�ⳣ������ƽ��������Ӧ������У�
��� �⣺��1����ѧƽ�ⳣ���Ĵ�Сֻ���¶��йأ������¶ȣ�ƽ�������ȵķ����ƶ�����Ӧ����ʼ����ͬ�����CH3OCH3��g�������ͣ�˵�����������ƶ�������ӦΪ���ȹ��̣����ԡ�HС��0��
�� 2CH3OH��g��=CH3OCH3��g��+H2O��g��
��ʼ��mol�� 0.2 0 0
20min��mol�� 0.16 0.08 0.08
ƽ�⣨mol�� 0.04 0.08 0.08
����v��CH2OH��=$\frac{��c}{��t}$=$\frac{\frac{0.16mol}{2L}}{20min}$=0.004mol•L-1•min-1��ƽ�ⳣ��K=$\frac{c��CH{\;}_{3}OCH{\;}_{3}��•c��H{\;}_{2}O��}{c{\;}^{2}��CH{\;}_{3}OH��}$=$\frac{0.08��0.08}{0.04{\;}^{2}}$=4��
�ʴ�Ϊ��С�ڣ���Ӧ����ʼ����ͬ�����CH3OCH3��g�������ͣ�˵�����������ƶ�������ӦΪ���ȹ��̡�H��0��0.004��4��
��2���������ƽ��ʱ��CH3OH������������������е���ͬ����Ϊ��Чƽ�⣬�ָ÷�ӦΪ�����������ķ�Ӧ������ֻҪ��Ӧ��ɱ�������Ч����ѹǿ�������ƽ��ʱ������������������Կ�ʼ��Ӧ��ҲӦ�������������������CH3OH��ʼ�����ʵ���Ϊ0.40mol��
��Чƽ������ʵ����������ͬ������ƽ��ʱCH3OCH3��g���������������������ͬ�����ݣ�1��������ʽ����������ƽ��ʱCH3OCH3��g�����������Ϊ$\frac{0.08}{0.08+0.08+0.04}$=0.4������������ƽ��ʱCH3OCH3��g�����������ҲΪ0.4��
�ʴ�Ϊ��0.40mol��0.4��
��3���¶�Խ�ͣ���Ӧ����ԽС���ﵽƽ��ʱ�����ʱ��Խ��������ȣ�����¶ȵͣ���Ӧ����ԽС����Ҫ��ʱ�䳤������t���Ӻ�������ﵽƽ�⣬t����20min�����������������������ݣ���ʼʱCH3OH��g��Ũ����ͬ��ƽ��ʱ������CH3OH��g��Ũ��Ϊ$\frac{0.20-0.08��2}{2}$=0.02mol/L��ƽ��ʱ������CH3OH��g��Ũ��Ϊ$\frac{0.20-0.09��2}{2}$=0.01mol/L������ͼ��Ϊ ��
��
�ʴ�Ϊ�����ڣ��¶�Խ�ͣ���Ӧ����ԽС���ﵽƽ��ʱ�����ʱ��Խ���� ��
��
��4��c��CH3OH��=$\frac{0.9}{3}$=0.3mol/L��c��CH3OCH3 ��=$\frac{0.6}{3}$=0.2mol/L��c��H2O��=$\frac{0.3}{3}$=0.1mol/L��Ũ����=$\frac{0.2��0.1}{0.3{\;}^{2}}$=$\frac{2}{9}$����Ϊ387��ʱ�÷�Ӧ��ƽ�ⳣ��K1=4���ֽ���ƽ��������Ӧ�����ƶ�������208��ʱƽ�ⳣ������4����Ũ����=$\frac{2}{9}$��4������ƽ��������Ӧ�����ƶ���v����v�����ʴ�Ϊ������
���� ���⿼���˻�ѧƽ�ⳣ�����йؼ����Լ���Чƽ���Ӧ�ã�����ƽ�ⳣ����ʽ����ƽ�ⳣ�����ٽ��Ũ���뷴Ӧ���ʵĹ�ϵ����ѧƽ�ⳣ����Ũ����֮��Ĺ�ϵ���������ע��÷�Ӧ�ص㣬��Ŀ�Ѷ��еȣ�

 ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д� Сѧ��ʱ��ҵȫͨ����ϵ�д�
Сѧ��ʱ��ҵȫͨ����ϵ�д�
| A�� | X����������ʯī | B�� | ���Ӵ�ͭ�缫�����·����X�缫 | ||
| C�� | Y������ͭ��Һ | D�� | X���ϵĵ缫��ӦʽΪAg++e-�TAg |
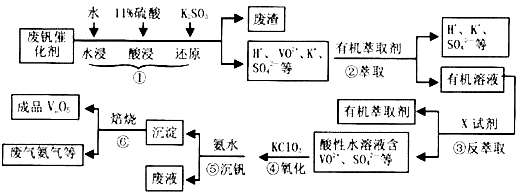
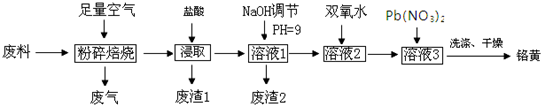
��1��������з�������Ҫ�ɷ���SiO2������X�Լ�ΪH2SO4��
��2���ڡ��۵ı仯���̿ɼ�ΪR2��SO4��n ��ˮ�㣩+2nHA���л��㣩?2RAn���л��㣩+nH2SO4��ˮ�㣩��R��ʾVO2+��HA��ʾ�л���ȡ������Ϊ��ߢ�����ȡ�ٷ��ʣ�Ӧ��ȡ�Ĵ�ʩ�Ǽ�����к�����ʹƽ�����ƣ�
��3������ɢ��з�Ӧ�����ӷ���ʽ��
ClO3-+6VO2++6H+�T6VO3++1Cl-+H2O��
��4��25��ʱ��ȡ����������������õ��������ʺ���ҺpH֮���ϵ���±���
| pH | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 | 2.0 | 2.1 |
| ��������/% | 88.1 | 94.8 | 96.5 | 98.0 | 98.8 | 98.8 | 96.4 | 93.1 | 89.3 |
��5���ù��������У�����ѭ�����õ��������л���ȡ����������
| A�� | 2015��2��15���𣬺���ʡ���������ա�������������������GB3095-2012��������ȫʡ14�����������ڵس��п���������������Ҫ��Ⱦ�����PM2.5��PM10��O3��CO2��SO2��NO2��6��ָ�� | |
| B�� | ���������������ڽ��ػ����������������ָ��£������������ǽ������� | |
| C�� | ����ˮ��������������ԵĽ������ӣ�������������ˮ��ɱ������ | |
| D�� | ��ʵ���ҵķ���Һ�ͷϼ�Һ�кͺ����ŷŷ��ϡ���ɫ��ѧ����Ҫ�� |
| A�� | ��ӷ� | B�� | ������ľ̿���� | C�� | ��������ˮ | D�� | ʳ������ˮ |
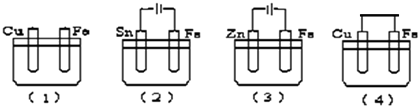
| A�� | ��3����4����2����1�� | B�� | ��3����1����4����2�� | C�� | ��4����3����1����2�� | D�� | ��3����4����1����2�� |
 ����������ѧ��Ӧԭ��֪ʶ����������⣺
����������ѧ��Ӧԭ��֪ʶ����������⣺