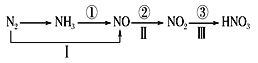
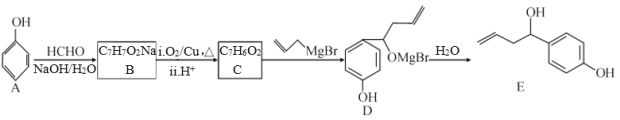
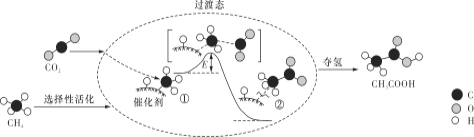
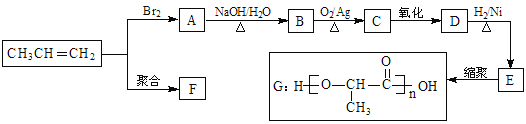
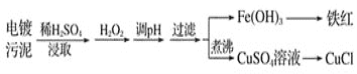
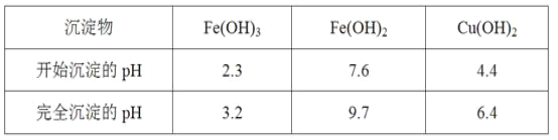
��Ŀ����
����Ŀ���ش��������⣺
(1)2 mol CO(NH2)2�к�_____molNԭ��, ����Oԭ������________g H2O������ԭ������ȡ�
(2)12.4 g Na2R��Na��0.4 mol����Na2R��Ħ������Ϊ_______����R������Ϊ1.6 g��Na2R�������ʵ���Ϊ_________��
(3)CO��CO2�Ļ������18 g���ڱ�״���µ����Ϊ11.2 L�����������ƽ��Ħ������Ϊ________�����������CO������Ϊ________��
���𰸡�4 36 62 g/mol 0.1 mol 36 g/mol 7 g
��������
(1)��ԭ�����ʵ���ΪCO(NH2)2��2����CO(NH2)2��H2O�����ж�����1��Oԭ�ӣ����ߺ�����ԭ����Ŀ��ȣ���������ʵ�����ȣ�
(2)�������������ʵ�������Na2R�����ʵ������ٸ���M��![]() ����Na2R��Ħ���������ٸ���R����������R�����ʵ�����
����Na2R��Ħ���������ٸ���R����������R�����ʵ�����
(3)����n��![]() ��
��![]() ��������������ļ��㣬����COΪxmol��CO2Ϊymol���з���ʽ���㡣
��������������ļ��㣬����COΪxmol��CO2Ϊymol���з���ʽ���㡣
(1)��ԭ�����ʵ���ΪCO(NH2)2��2����2mol CO(NH2)2�к���ԭ�����ʵ�����2mol��2��4mol��CO(NH2)2��H2O�����ж�����1��Oԭ�ӣ����ߺ�����ԭ����Ŀ��ȣ���������ʵ�����ȣ���ˮ�����ʵ���Ϊ2mol������Ϊ36g���ʴ�Ϊ��4��36��
(2)12.4gNa2R��Na�������ʵ���Ϊ0.4mol����Na2R�����ʵ���Ϊ0.4mol/2��0.2mol��Na2R��Ħ������Ϊ![]() ��62g/mol��Na2R����Է�������Ϊ62��R�����ԭ��������6223��2��16����R������Ϊ1.6 g��Na2R�У�R�����ʵ���Ϊ
��62g/mol��Na2R����Է�������Ϊ62��R�����ԭ��������6223��2��16����R������Ϊ1.6 g��Na2R�У�R�����ʵ���Ϊ![]() =0.1mol��Na2R�����ʵ�����R��ͬ��ҲΪ0.1mol���ʴ�Ϊ��62g/mol��0.1mol��
=0.1mol��Na2R�����ʵ�����R��ͬ��ҲΪ0.1mol���ʴ�Ϊ��62g/mol��0.1mol��
(3)��������ʵ���Ϊn��![]() ��
��![]() ��0.5mol��ƽ��Ħ������ΪM=
��0.5mol��ƽ��Ħ������ΪM=![]() =
=![]() =36g/mol����COΪxmol��CO2Ϊymol����28x��44y��18��x��y��0.5����֮�ã�x��0.25��y��0.25��m(CO)��0.25mol��28g/mol��7g���ʴ�Ϊ��36g/mol��7g��
=36g/mol����COΪxmol��CO2Ϊymol����28x��44y��18��x��y��0.5����֮�ã�x��0.25��y��0.25��m(CO)��0.25mol��28g/mol��7g���ʴ�Ϊ��36g/mol��7g��

����Ŀ��ijͬѧ��������������������ᷴӦ���й�ʵ�飬ʵ����̵����ݼ�¼���£����������ϱ�����Ϣ���ش��й����⣺
ʵ����� | ��Ӧ�¶�/ | �μӷ�Ӧ������ | ||||
|
|
| ||||
|
|
|
|
| ||
A | 20 | 10 | 0.1 | 10 | 0.1 | 0 |
B | 20 | 5 | 0.1 | 10 | 0.1 | 5 |
C | 20 | 10 | 0.1 | 5 | 0.1 | 5 |
D | 40 | 5 | 0.1 | 10 | 0.1 | 5 |
��1�������������յ�֪ʶ�жϣ�������ʵ���У���Ӧ��������ʵ�������__________����ʵ����ţ���
��2���ڱȽ�ijһ���ض�ʵ�������Ӱ��ʱ�������ų��������صı䶯���ţ�����Ҫ���ƺ���ʵ���йصĸ��Ӧ���������У�
����˵���¶ȶԸ÷�Ӧ����Ӱ��������__________����ʵ����ţ���
��A��B��A��C����ϱȽϣ����о���������___________________________________________��
��B��C����ϱȽϣ����о���������___________________________________________��
��3��ʵ���������˳��ֻ�ɫ�����Ŀ������ȽϷ�Ӧ���ʵĿ������������Ϊ�β����ò�����λʱ���ڲ�����������Ĵ�С���бȽϣ�___________________________________________��