��Ŀ����
19����֪�����²��0.01mol•L-1 H2SO4��Һ�У�c��H+��=0.011mol•L-1���ݴ˻ش��������⣺��1������ĵ��뷽��ʽΪH2SO4=2H++SO42-��0.1mol•L-1 NaHSO4��Һ�и���Ũ���ɴ�С��˳��Ϊc��Na+����c��HSO4-����c��H+����c��SO42-����c��OH-����������������ƶ�Na2SO4��Һ�ʼ�����ᡱ���С����ԣ�ԭ���ǣ������ӷ���ʽ��ʾ��SO42-+H2O?OH-+HSO4-��
��2�������£�0.01mol•L-1��NaHSO4��Һ��c��H+����0.001mol•L-1�����������������=������
��3��������������ƶ�2mol•L-1��NaHSO4��Һ��1mol•L-1��Ba��OH��2��Һ�������ϣ���Ӧ�����ӷ���ʽΪ2OH-+2HSO4-+Ba2+=BaSO4��+2H2O+SO42-��
���� ������0.01mol•L-1 H2SO4��ǿ�ᣩ��Һ��c��H+��=0.011mol•L-1��˵������ĵڶ���������ڵ���ƽ�⣬
��1��������Һ����ȫ���룬����������ӵĵ���̶ȴ�����ˮ��̶ȣ���Һ��ʾ���ԣ��ݴ��жϸ�����Ũ�ȴ�С����������Һ�У���������Ӳ���ˮ����������������ӣ���Һ��ʾ���ԣ��ݴ�д����ˮ�ⷽ��ʽ��
��2������ĵ�һ��������������ڶ������룬��������������������ӵĵ���̶ȼ�С���ݴ��ж�0.01mol/L������������Һ��������Ũ�ȴ�С��
��3����������������������Ӧ�������ᱵ��ˮ�������������Ʋ��ܲ���������ӹ����������μ�����������ʣ�������������뱵���ӷ�Ӧ�������ᱵ������
��� �⣺������0.01mol•L-1 H2SO4��ǿ�ᣩ��Һ��c��H+��=0.011mol•L-1��˵����һ����������ȫ�ģ�H2SO4=H++HSO4-���ڶ������벢����ȫ��HSO4-?H++SO42-��
��1��������Һ�е��뷽��ʽΪ��H2SO4=2H++SO42-��NaHSO4��Һ�У�����������ӵĵ���̶ȴ�����ˮ��̶ȣ���Һ��ʾ���ԣ���c��H+����c��OH-����c��Na+����c��HSO4-������Һ������Ũ�ȴ�СΪ��c��Na+����c��HSO4-����c��H+����c��SO42-����c��OH-����
Na2SO4��Һ�������������ˮ�⣬������Һ�д���ˮ��ƽ�⣺SO42-+H2O?OH-+HSO4-��������Һ�������ԣ�
�ʴ�Ϊ��H2SO4=2H++SO42-��c��Na+����c��HSO4-����c��H+����c��SO42-����c��OH-�����SO42-+H2O?OH-+HSO4-��
��2��0.01mol•L-1 H2SO4��ǿ�ᣩ��Һ��c��H+��=0.011mol•L-1������ĵ�һ�������������Ũ��Ϊ0.01mol/L����ڶ����������������Ϊ0.001mol/L������ĵ�һ������������������Ƶڶ������룬����0.01mol/L������������Һ�У�������Ũ��Ӧ�ô���0.001mol/L��
�ʴ�Ϊ������
��3��2mol•L-1 NaHSO4��Һ��1mol•L-1 Ba��OH��2��Һ�������ϣ���Ӧ����������ӹ�������Ӧ�����ӷ���ʽΪ��2OH-+2HSO4-+Ba2+=BaSO4��+2H2O+SO42-���������μ�Ba��OH��2��Һ������������������ӷ�Ӧ�������ᱵ���������ӷ���ʽΪ��SO42-+Ba2+=BaSO4����
�ʴ�Ϊ��2OH-+2HSO4-+Ba2+=BaSO4��+2H2O+SO42-��
���� ���⿼��������Ũ�ȴ�С�Ƚϡ��ε�ˮ��ԭ������Ӧ�ã���Ŀ�Ѷ��еȣ���ȷ����ĵڶ������ֵ���Ϊ���ؼ���ע�������ж�����Ũ�ȴ�С���÷�����������ؿ���ѧ���ķ���������������

 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д� Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д�| A�� | HCl��HBr��HI���ۡ��е�������������Ӽ���������С�й� | |
| B�� | H2O���ۡ��е����H2S������H2O����֮�������� | |
| C�� | �������ˮ�γ���� | |
| D�� | I2������CCl4��������������ԭ������ |
| �����ܣ�kJ/mol�� | I1 | I2 | I3 | I4 |
| A | 932 | 1821 | 15390 | 21771 |
| B | 738 | 1451 | 7733 | 10540 |
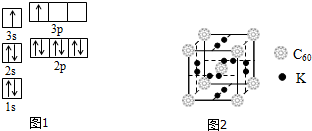
��2��ACl2������A���ӻ�����Ϊsp�ӻ���
��3��������Ϊһ�������Դ�����������Ĵ������⣬C60������������ϣ���֪���ʯ�е�C-C�ļ���Ϊ154.45pm��C60��C-C����Ϊ145-140pm����ͬѧ�ݴ���ΪC60���۵���ڽ��ʯ������Ϊ�˹۵��Ƿ���ȷ����ȷ�����ȷ������ȷ�����������������жϵ����ɣ�C60�Ƿ��Ӿ��壬�ۻ�ʱ�����ƻ���ѧ����
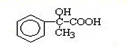
��4����ѧ�Ұ�C60�ͼز�����һ��������һ�ָ���ϩ������侧����ͼ2��ʾ���������ڵ���ʱ��һ�ֳ����壮д����̬��ԭ�ӵļ۵����Ų�ʽ4S1����������Kԭ�Ӻ�C60���ӵĸ�����Ϊ3��1��
��5��C��Si��Nԭ�ӵ縺���ɴ�С��˳����N��C��Si��NCl3���ӵ�VSEPRģ��Ϊ�������壮
| A�� | NaOH��ϡH2SO4��OH-+H+�TH2O | |
| B�� | NH4HCO3��Һ�����NaOH��Һ��Ӧ��NH4++OH-�TNH3��+H2O | |
| C�� | ̼������Һ��ͨ������CO2��CO32-+CO2+H2O�T2HCO3- | |
| D�� | NaHCO3��H2SO4��Ӧ��HCO3-+H+�TH2O+CO2�� |
| A�� | �������� W���淴Ӧ�������� | |
| B�� | �����¶ȣ�ƽ�������ƶ� | |
| C�� | ƽ������ X��������Ӧ�ġ�H���� | |
| D�� | ������������ѹǿ����ʱ����Ӧ�ﵽƽ�� |

 �����ϳ�A����ϳ�·�����£���֪��A������������ˮ�������л���B�ͼ״���
�����ϳ�A����ϳ�·�����£���֪��A������������ˮ�������л���B�ͼ״���
 ��
��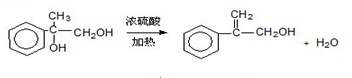 ��
��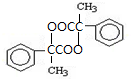 ��
��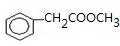 ��
��