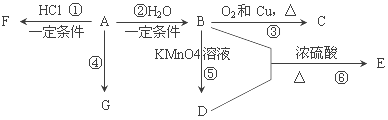
题目内容
【题目】现有常温下pH=2的HCl溶液甲和pH=2的CH3COOH溶液乙,请根据下列操作回答问题:
(1)常温下0.1mol·L-1的CH3COOH溶液加水稀释过程中,下列表达式的数据一定变小的是_____。
A.c(H+) B. c(H+)/c(CH3COOH) C.c(H+)·c(OH-)
(2)取10mL的乙溶液,加入等体积的水,CH3COOH的电离平衡________(填“向左”“向右”或“不”)移动;另取10mL的乙溶液,加入少量无水醋酸钠固体(假设加入固体前后溶液体积保持不变),待固体溶解后,溶液中的 c(H+)/c(CH3COOH) 比值将________(填“增大”“减小”或“无法确定”)。
(3)取等体积的甲、乙两溶液,分别用等浓度的NaOH稀溶液中和,则消耗NaOH溶液体积的大小关系为V(甲)______(填“>”“<”或“=”)V(乙)。
(4)已知25℃时,下列酸的电离平衡常数如下:
化学式 | CH3COOH | H2CO3 | HClO | H2SO3 |
电离平衡常数 | 1.8×10-5 | K1=4.3×10-7 K2=4.7×10-11 | 3.0×10-8 | K1=1.54×10-2 K2=1.02×10-7 |
①下列微粒可以大量共存的是______![]() 填字母
填字母![]() 。
。
a.CO32-、HSO3- b.HCO3-、HSO3-
c.SO32-、HCO3- d.CO32- 、H2CO3
②写出下列反应的离子方程式:
H2SO3+Na2CO3(少量):_______________
室温下,0.1 mol·L-l的KOH溶液滴10.00mL 0.10 mol·L-l H2C2O4 (二元弱酸)溶液,所得滴定曲线如图(混合溶液的体积可看成混合前溶液的体积之和)。请回答下列问题:
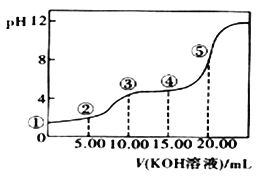
(5)点④所示溶液中:c(K+)+c(H2C2O4)+c(HC2O4)+c(C2O42)=_____mol/L。点⑤所示的溶液中各离子浓度的大小顺序_____.
(6)草酸晶体(H2C2O4·2H2O)为无色,某同学设计实验测定其纯度。实验过程如下:称取mg草酸晶体于锥形瓶中,加水完全溶解用cmol·L-1酸性KMnO4标准溶液进行滴定,则达到滴定终点时的现象是_______;该过程中发生反应的离子方程式为________;滴定过程中消耗VmLKMnO4标准溶液,草酸晶体纯度为________。
【答案】A 向右 减小 < bc H2SO3+CO32-=== HSO3-+HCO3- 0.10 c(K+)>c(C2O42-)>c(OH-)>c(HC2O4-)>c(H+) 加入最后一滴高锰酸钾溶液,锥形瓶内溶液由无色变为红色,且半分钟不褪色 5H2C2O4+2MnO4-+6H+=2Mn2++10CO2↑+8H2O 0.315cv/m
【解析】
(1)CH3COOH溶液加水稀释过程,促进电离,n(H+)增大,酸性减弱c(H+)减小,c(OH-)增大,Kw不变;
(2)醋酸是弱电解质,加水稀释促进醋酸电离;向醋酸中加入醋酸钠固体,溶液中醋酸根离子浓度增大,抑制醋酸电离;
(3)pH相等的醋酸和盐酸,醋酸的浓度大于盐酸,等体积等pH的两种酸,醋酸的物质的量大于盐酸,酸的物质的量越多需要等浓度的氢氧化钠溶液体积越大;
(4)①根据Ka越大酸性越强,根据酸性较强的能与酸性较弱的酸根离子反应;
②H2SO3和Na2CO3反应生成NaHSO3和NaHCO3;
(5)根据点④所示的溶液的体积25mL计算出溶液中各组分的浓度;根据点⑤所示的溶液中,溶质只有K2C2O4分析各离子浓度的大小;
(6)用cmolL-1酸性KMnO4标准溶液进行滴定,利用高锰酸钾溶液的颜色指示反应终点,高锰酸钾溶液氧化草酸生成二氧化碳,结合反应的定量关系计算草酸晶体纯度。
(1)A.CH3COOH溶液加水稀释过程,促进电离,c(H+)减小,故A正确;
B.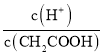 =
=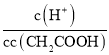 ×
×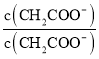 =
=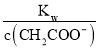 ,K不变,醋酸根离子浓度减小,则稀释过程中比值变大,故B错误;
,K不变,醋酸根离子浓度减小,则稀释过程中比值变大,故B错误;
C.稀释过程,促进电离,c(H+)减小,c(OH-)增大,c(H+)c(OH-)=Kw,Kw不变,故C错误;
故答案为A;
(2)醋酸是弱电解质,加水稀释促进醋酸电离,所以醋酸电离平衡向正反应方向移动;向醋酸中加入醋酸钠固体,溶液中醋酸根离子浓度增大,抑制醋酸电离,则氢离子浓度减小,醋酸分子浓度增大,所以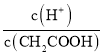 减小;
减小;
(3)pH相等的醋酸和盐酸,醋酸的浓度大于盐酸,等体积等pH的两种酸,醋酸的物质的量大于盐酸,酸的物质的量越多需要等浓度的氢氧化钠溶液体积越大,所以消耗的NaOH溶液的体积大小关系为:V(甲)<V(乙);
(4)①已知Ka越大酸性越强,酸性强弱顺序为H2SO3>CH3COOH>H2CO3>HSO3->HClO>HCO3-,且酸性较强的能与酸性较弱的酸根离子反应,由于HCO3-的酸性小于HSO3-的酸性,所以HCO3-与HSO3-、SO32-不反应,即bc能共存,故答案为bc;
②H2SO3和Na2CO3反应生成NaHSO3和NaHCO3,发生反应的离子方程式为H2SO3+CO32-=== HSO3-+HCO3-;
(5)点④所示的溶液的体积25mL,根据物料守恒:c(HC2O4-)+c(H2C2O4)+c(C2O42-)=0.10mol/L×![]() =0.04mol/L;c(K+)=0.10mol/L×
=0.04mol/L;c(K+)=0.10mol/L×![]() =0.06mol/L,所以c(HC2O4-)+c(H2C2O4)+c(C2O42-)+c(K+)=0.10molL-1;点⑤所示的溶液中,溶质只有K2C2O4,水解后溶液显示碱性,离子浓度大小关系为:c(K+)>c(C2O
=0.06mol/L,所以c(HC2O4-)+c(H2C2O4)+c(C2O42-)+c(K+)=0.10molL-1;点⑤所示的溶液中,溶质只有K2C2O4,水解后溶液显示碱性,离子浓度大小关系为:c(K+)>c(C2O
(6)称取m g草酸晶体于试管中,加水完全溶解用cmolL-1酸性KMnO4标准溶液进行滴定,则达到滴定终点时的现象是:当滴入最后一滴标准液时,溶液由无色变成紫红色,且半分钟内溶液颜色不再改变,该过程中发生反应的离子方程式为:2MnO4-+6H++5H2C2O4=2Mn2++10CO2↑+8H2O,滴定过程中消耗V mL KMnO4标准溶液,结合离子方程式定量关系计算;
2MnO4-+6H++5H2C2O4=2Mn2++10CO2↑+8H2O
2 5
cmolL-1×V×10-3L n
n=2.5cV×10-3mol,草酸晶体H2C2O42H2O的纯度=![]() ×100%=
×100%=![]() %。
%。

 阅读快车系列答案
阅读快车系列答案