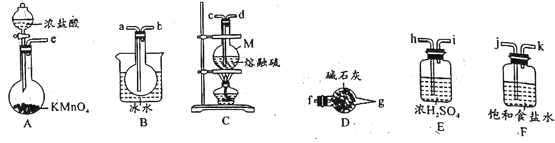
��Ŀ����
����Ŀ����֪��A��ʯ���ѽ�������Ҫ����֮һ������������ں���һ������ʯ�ͻ�����չˮƽ�ı�־���������л��� A��G֮���ת����ϵ��
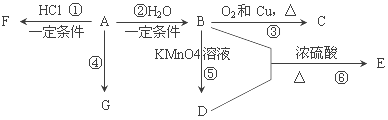
��ش��������⣺
(1)A�Ĺ����ŵ�������_________��C�Ľṹ��ʽ��_____��
(2)B��һ�־���������ζ��Һ�壬��A��B�ķ�Ӧ����ʽΪ_____���÷�Ӧ������_____��
(3)G ��һ�ָ߷��ӻ������ṹ��ʽ��_____��
(4)�����У����˶�Ա������˻�Ť��ʱ�����ҽ���������˲�λ��������F(�е�12.27��C)����Ӧ��������д����A��F�Ļ�ѧ��Ӧ����ʽ_____��
(5)D�Ľṹ��ʽΪ_____��
���𰸡�̼̼˫�� CH3CHO CH2=CH2+H2O![]() CH3CH2OH �ӳɷ�Ӧ
CH3CH2OH �ӳɷ�Ӧ ![]() CH2��CH2+HCl
CH2��CH2+HCl![]() CH3CH2Cl CH3COOH
CH3CH2Cl CH3COOH
��������
A��ʯ���ѽ�������Ҫ����֮һ������������ں���һ��ʯ�ͻ�����չˮƽ�ı�־����A����ϩ��
��ϩ����̼̼˫����ˮ�����ӳɷ�Ӧ�����Ҵ�����B���Ҵ���
�Ҵ�����������������ȩ����C����ȩ��
�Ҵ������������Һ���������ᣬ������Ҵ�����������Ӧ����������������D�����ᣬE������������
��ϩ��HCl��һ�������·����ӳɷ�Ӧ���������飬��FΪ�����飻
��ϩ��һ�������·����Ӿ۷�Ӧ���ɾ���ϩ������ϩ�Ǹ߷��ӻ������GΪ����ϩ���ݴ˽��
(1)AΪ��ϩ����ϩ�Ĺ����ŵ�������̼̼˫����CΪ��ȩ����ȩ�Ľṹ��ʽ��CH3CHO���ʴ�Ϊ��̼̼˫����CH3CHO��
(2)BΪ�Ҵ�����ϩ��ˮ�����ӳɷ�Ӧ�����Ҵ�����Ӧ�Ļ�ѧ����ʽΪ��CH2=CH2+H2O![]() CH3CH2OH���ʴ�Ϊ��CH2=CH2+H2O
CH3CH2OH���ʴ�Ϊ��CH2=CH2+H2O![]() CH3CH2OH���ӳɷ�Ӧ��
CH3CH2OH���ӳɷ�Ӧ��
(3)GΪ����ϩ���ṹ��ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(4)AΪ��ϩ����ϩ��HCl�����ӳɷ�Ӧ����F(������)��������Ӧ�Ļ�ѧ����ʽΪ��CH2��CH2+HCl![]() CH3CH2Cl���ʴ�Ϊ��CH2��CH2+HCl
CH3CH2Cl���ʴ�Ϊ��CH2��CH2+HCl![]() CH3CH2Cl��
CH3CH2Cl��
(5)DΪ���ᣬ�ṹ��ʽΪCH3COOH���ʴ�Ϊ��CH3COOH��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�����г�����pH��2��HCl��Һ��pH��2��CH3COOH��Һ�ң���������в����ش����⣺
��1��������0.1mol��L��1��CH3COOH��Һ��ˮϡ�����У����б���ʽ������һ����С����_____��
A��c(H��) B�� c(H+)/c(CH3COOH) C��c(H��)��c(OH��)
��2��ȡ10mL������Һ������������ˮ��CH3COOH�ĵ���ƽ��________(��������������������������)�ƶ�����ȡ10mL������Һ������������ˮ�����ƹ���(����������ǰ����Һ������ֲ���)���������ܽ����Һ�е� c(H+)/c(CH3COOH) ��ֵ��________(��������������С��������ȷ����)��
��3��ȡ������ļס�������Һ���ֱ��õ�Ũ�ȵ�NaOHϡ��Һ�кͣ�������NaOH��Һ����Ĵ�С��ϵΪV(��)______(����������������������)V(��)��
��4����֪25��ʱ��������ĵ���ƽ�ⳣ�����£�
��ѧʽ | CH3COOH | H2CO3 | HClO | H2SO3 |
����ƽ�ⳣ�� | 1.8��10��5 | K1��4.3��10��7 K2��4.7��10��11 | 3.0��10��8 | K1��1.54��10��2 K2��1.02��10��7 |
�����������Դ����������______![]() ����ĸ
����ĸ![]() ��
��
a.CO32-��HSO3- b.HCO3-��HSO3-
c.SO32-��HCO3- d.CO32- ��H2CO3
��д�����з�Ӧ�����ӷ���ʽ��
H2SO3��Na2CO3(����)��_______________
�����£�0.1 mol��L��l��KOH��Һ��10.00mL 0.10 mol��L��l H2C2O4 (��Ԫ���ᣩ��Һ�����õζ�������ͼ(�����Һ������ɿ��ɻ��ǰ��Һ�����֮��)����ش��������⣺
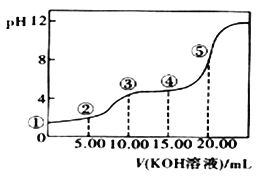
��5�������ʾ��Һ�У�c(K+)+c(H2C2O4)+c(HC2O4)+c(C2O42)��_____mol/L�������ʾ����Һ�и�����Ũ�ȵĴ�С˳��_____��
��6�����ᾧ��(H2C2O4��2H2O)Ϊ��ɫ��ijͬѧ���ʵ��ⶨ�䴿�ȡ�ʵ��������£���ȡmg���ᾧ������ƿ��,��ˮ��ȫ�ܽ���cmol��L-1����KMnO4����Һ���еζ�����ﵽ�ζ��յ�ʱ��������_______���ù����з�����Ӧ�����ӷ���ʽΪ________���ζ�����������VmLKMnO4����Һ�����ᾧ�崿��Ϊ________��
����Ŀ������ݱ������ṩ�ļס��ҡ�����������Ԫ�ص������Ϣ����������⣺
Ԫ�� | �� | �� | �� | �� |
ԭ������ | 11 | �� | ||
Ԫ�ط��� | �� | S | ||
ԭ�ӽṹʾ��ͼ | �� |
| ||
Ԫ�����ڱ��е�λ�� | �������� ��A�� | �� |
��1����д�����и��������Ӧ�Ŀհף���_________________��__________________
��_________________��_________________��
��2���ס��ҡ�����������Ԫ���У�ԭ�Ӱ뾶������_______����Ԫ�ط��ţ�����Ԫ�ص��������Ϊ____��(��ϼ�)��
��3������������Ӧˮ����ļ��ԣ���_______________�ң�������������������������̬�⻯����ȶ��ԣ���_______________������������������������
��4��Ԫ���ҵ�����������Ӧˮ���������Ԫ�ؼ�����������Ӧˮ���ﷴӦ�������к����θ�ᡣ
��ֱ�д��������Ӧ�����ӷ���ʽ��_____________��______________��
��5��������ͨ�������ʯ���п��Ƶ�Ư�ۣ�д���÷�Ӧ�Ļ�ѧ����ʽ��_____________________��