��Ŀ����
����Ŀ��������������ȷ���ǣ� ��
A. 1 molN2��4molH2��ϳ�ַ�Ӧ����ת�Ƶĵ�����ĿΪ6mol
B. ��0.1mol��L��1CH3COONa��Һ�м�������ˮ����Һ��![]() ��С
��С
C. �����£�C(s)��H2O(g)![]() CO(g)��H2(g)�����Է����У���÷�Ӧ����H>0
CO(g)��H2(g)�����Է����У���÷�Ӧ����H>0
D. HCl��NaOH��Ӧ���к���Ϊ��57.3kJ��mol��1����H2SO4��Ba(OH)2��Ӧ���к���Ϊ��114.6 kJ��mol��1
���𰸡�C
��������
A�������������ķ�ӦΪ���淴Ӧ�����ܽ��г��ף�1 molN2��4molH2��ϳ�ַ�Ӧ��ת�Ƶĵ�����С��6mol����A����
B����0.1molL-1CH3COONa��Һ�м�������ˮ���ٽ�CH3COO-��ˮ�⣬��Һ��c��OH-����С��������Ũ�������������Ũ�ȼ�С������Һ��![]() �ı�ֵ����B����
�ı�ֵ����B����
C�������£���ӦC(s)+H2O(g)CO(g)+H2(g) �����Է����У���S��0����H-T��S��0����÷�Ӧ����H��0����C��ȷ��
D��HCl��NaOH��Ӧ���к�����H=-57.3kJ/mol������ϡǿ����ǿ�Ӧ����1molҺ̬ˮ�ų�57.3kJ����������H2SO4��Ba(OH)2��Ӧ���к���һ�������ָ����1molˮ�ų�����������һ������������������������Ӧʱ���г�����Ӧ�ų���������D����
��ѡC��

����Ŀ��BaCl2�����ڵ��ӡ��DZ��ȹ�ҵ���Զ���ʯ(��Ҫ�ɷ�ΪBaCO3����������CaCO3��MgSO4��Fe2O3��SiO2������)Ϊԭ�ϣ�ģ�ҵ��ȡBaCl2��2H2O����������ͼ��ʾ��
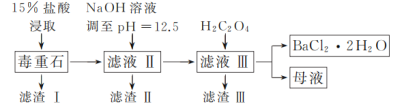
��֪��Ksp(BaC2O4)��1.6��10��7��Ksp(CaC2O4)��2.3��10��9��
Ca2�� | Mg2�� | Fe3�� | |
��ʼ����ʱ��pH | 11.9 | 9.1 | 1.9 |
��ȫ����ʱ��pH | 13.9 | 11.0 | 3.7 |
��1�������ȡʱ��Ҫ�ʵ����ȵ�ԭ����_____��
��2��������ijɷ�Ϊ_____��
��3������H2C2O4ʱӦ�����������ԭ����_____��
��4����37%����������15%���������õ���������_____(����ĸ)��
A����Ͳ B���ձ� C������ƿ D��������
��5���������辭��ϴ�Ӻ���ܽ��к����ӹ�������ԭ����_____��
��6���벹��������̼�ᱵʯ(��30%CaCO3��BaCO3��ʯ)ģ�ҵ��ȡBaCl2��2H2O��ʵ�鲽��Ϊ_____�����ˣ�����ˮ�Ҵ�ϴ��2��3�Σ����¸��(ʵ���п�ʹ�õ��Լ��У���ˮ��80����ˮ��0.1mol��L��1���ᣬ6mol��L��1����)