��Ŀ����
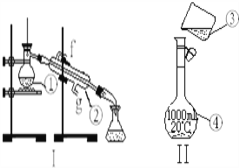
����Ŀ����ͼΪʵ����ijŨ�����Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺

��1����Ũ������HCl�����ʵ���Ũ��Ϊ_______mol��L-1 ��
��2��ȡ����������ĸ�������Һʱ�������������в�����ȡ��
���Ķ��ٶ��仯���� ______ ��
A����Һ��HCl�����ʵ��� B����Һ��Ũ��
C����Һ��Cl-����Ŀ D����Һ���ܶ�
��3��ijѧ����������Ũ���������ˮ����500 ml ���ʵ���Ũ��Ϊ0.400 mol��L-1ϡ���ᡣ
�ٸ�ѧ����Ҫ��ȡ________ ml ����Ũ����������ơ�
������ʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ�_________________��
A����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
B������Ͳȷ��ȡ����Ũ���ᵹ���ձ��У�Ȼ���������ˮ��Լ30mL�������ò������������裬ʹ���Ͼ���
C��������ȴ�������ز�����ע��500mL������ƿ��
D��������ƿ�ǽ����ߵ�ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm��
�������ƹ����У�����ʵ������������Ƶ�ϡ��������ʵ���Ũ���к�Ӱ�죿���ں���������ƫ�ߡ�����ƫ�͡�������Ӱ�족����
I������Ͳ��ȡŨ����ʱ���ӹ۲�_____________
II�����ݺ���ҡ�ȡ����ã�����Һ���½����ټ�����������ˮ______
����ʱ�����ӿ̶���________________
���𰸡� 11.9 BD 16.8 BCAFED ƫ�� ƫ�� ƫ��
��������(1)��������36.5%���ܶ�1.19g/mL Ũ��������ʵ���Ũ��=![]() mol/L=11.9mol/L���ʴ�Ϊ��11.9��
mol/L=11.9mol/L���ʴ�Ϊ��11.9��
(2)��Һ�Ǿ��ȵģ���Һ���ܶȡ�Ũ�Ȳ�������仯����HCl�����ʵ�����Cl-����Ŀ����Һ����йأ��ʴ�Ϊ��BD��
(3)������ҪŨ��������ΪV mL������ϡ�Ͷ��ɣ�ϡ��ǰ��HCl�����ʵ������䣬��V��10-3L��11.9mol/L=0.5 L��0.400mol/L�����V=16.8���ʴ�Ϊ��16.8��
������һ�����ʵ���Ũ�ȵ���Һ����Ϊ����ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�������ȷ�IJ���˳��Ϊ��BCAFED���ʴ�Ϊ��BCAFED��
����.����Ͳ��ȡŨ����ʱ���ӹ۲찼Һ�棬������ȡ��Ũ��������ƫС�����Ƶ���ҺŨ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
��.���ݺ���ҡ�ȡ����ã�����Һ���½����ټ�����������ˮ���������Ƶ���Һ���ƫ�����Ƶ���ҺŨ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
��.����ʱ���ӿ̶��ߣ����¼��������ˮ����������ƿ�̶��ߣ����Ƶ���Һ���ƫ�����Ƶ���ҺŨ��ƫ�ͣ��ʴ�Ϊ��ƫ����

 ��У����ϵ�д�
��У����ϵ�д�